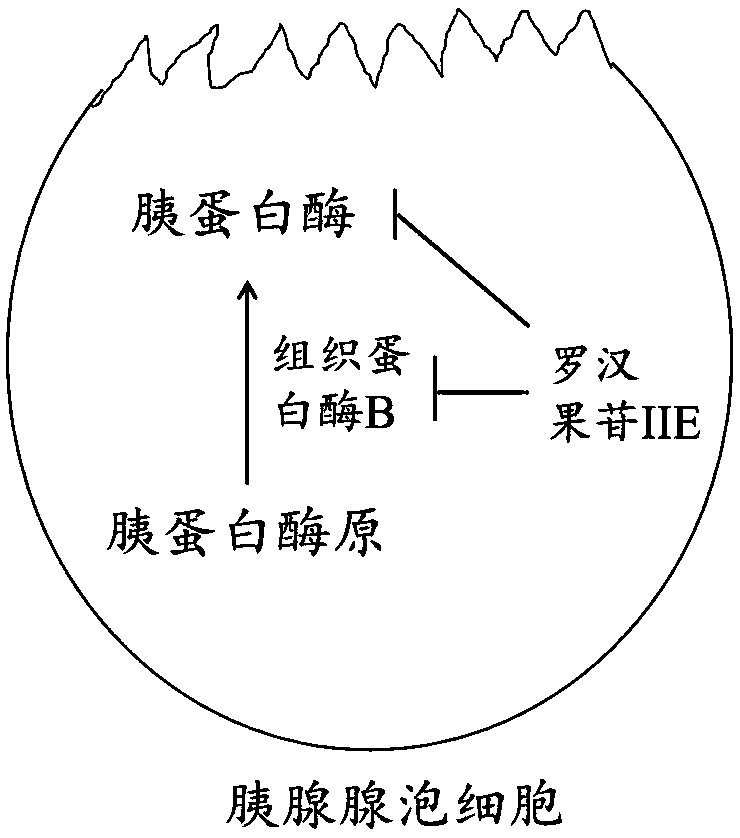
Application of mogroside IIE in preparing trypsin inhibitors
A technology of trypsin inhibition and mogroside, which is applied in the field of medicine, can solve problems such as insufficient research, and achieve the effects of less toxic side effects, wide sources, and good bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Time-effect experiment of mogroside IIE acting on AR42J cells
[0051] Rat pancreatic exocrine cell AR42J cells were purchased from the ATCC cell bank in the United States. AR42J culture conditions are: F12K medium (ATCC) and 20% fetal bovine serum (Gibco), 37°C, 5% carbon dioxide. Cell characteristics: Rat origin, adherent growth, epithelial cells, no mycoplasma, sterile.
[0052] Purchase Mogroside IIE at Chengdu Master Biotechnology Co., Ltd., in powder form, molecular weight 801, molecular formula C 42 h 72 o 14 , purity ≥ 98% (HPLC), soluble in methanol and dimethyl sulfoxide. The structural formula of the mogroside IIE is:
[0053]
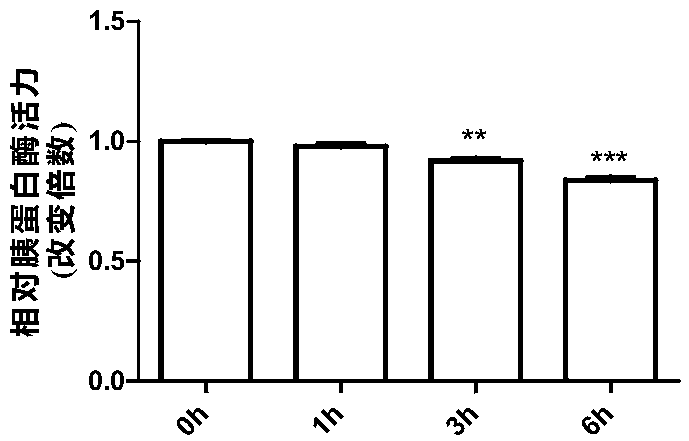
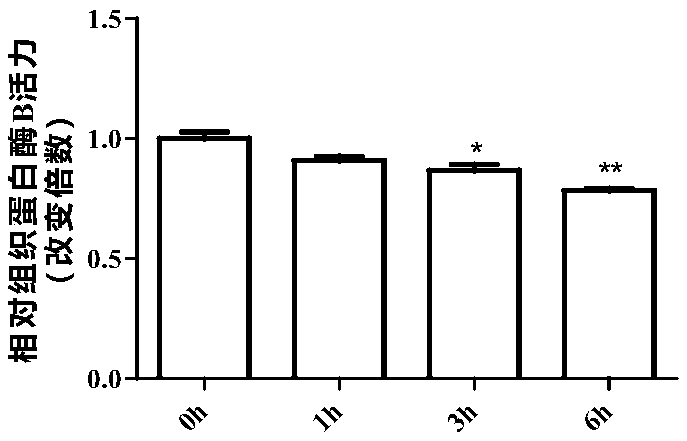
[0054] In rat pancreatic exocrine cell AR42J cells, 20 μM mogroside IIE (dissolved in methanol in Examples 1-6) was added. After treatment for 0h, 1h, 3h and 6h, respectively, the activities of trypsin and cathepsin B in AR42J cells were detected.
[0055] Wherein, trypsin activity assay (the following compounds a...
Embodiment 2
[0062] Example 2: Time-effect experiment of mogroside IIE acting on primary pancreatic acinar cells
[0063] In mouse primary pancreatic acini, 20 μM mogroside IIE was added. After being treated for 0h, 1h, 3h and 6h, respectively, the activities of trypsin and cathepsin B in the cells were detected.
[0064] Depend on Figure 4 It can be seen that the trypsin activity decreased by 1.21%, 7.4% and 15.21% after mogroside IIE was treated for 1h, 3h and 6h respectively.
[0065] Depend on Figure 5 It can be seen that the activity of cathepsin B decreased by 10.13%, 13.74% and 18.26% after mogroside IIE was treated for 1h, 3h and 6h respectively.
[0066] It can be seen that mogroside IIE inhibits the activity of trypsin and cathepsin B in primary pancreatic acinar cells in a time-dependent manner.
Embodiment 3
[0067] Example 3: Concentration Effect Experiment of Mogroside IIE on AR42J Cells
[0068] In rat pancreatic exocrine cell AR42J cells, 0 μM, 5 μM, 10 μM and 20 μM mogroside IIE were added, respectively. After being treated for 6 hours, the activities of trypsin and cathepsin B in the cells were detected.
[0069] Depend on Figure 6 It can be seen that after the action of 5 μM, 10 μM and 20 μM mogroside IIE, the trypsin activity decreased by 8.57%, 18.8% and 20.89% respectively.
[0070] Depend on Figure 7 It can be seen that after the action of 5 μM, 10 μM and 20 μM mogroside IIE, the activity of cathepsin B decreased by 12.19%, 21.3% and 26.42%, respectively.
[0071] It can be seen that mogroside IIE inhibits the activity of trypsin and cathepsin B in AR42J cells in a concentration-dependent manner.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com