Cell line capable of stably expressing 6His-Nav1.1 fusion protein and construction
A technology of 6his-nav1.1 and fusion protein, which is applied in the direction of peptides containing His tags, genetically modified cells, animal/human proteins, etc., to achieve the effect of easy purification and enrichment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment provides a 6His-tagged NaV1.1 fusion protein expression cell line and its construction method, comprising the following steps:
[0034] NaV1.1 gene information: human NaV1.1 (SCN1A), NM_001165963, CDS size is 6030bp. As shown in SEQID No.1:
[0035]
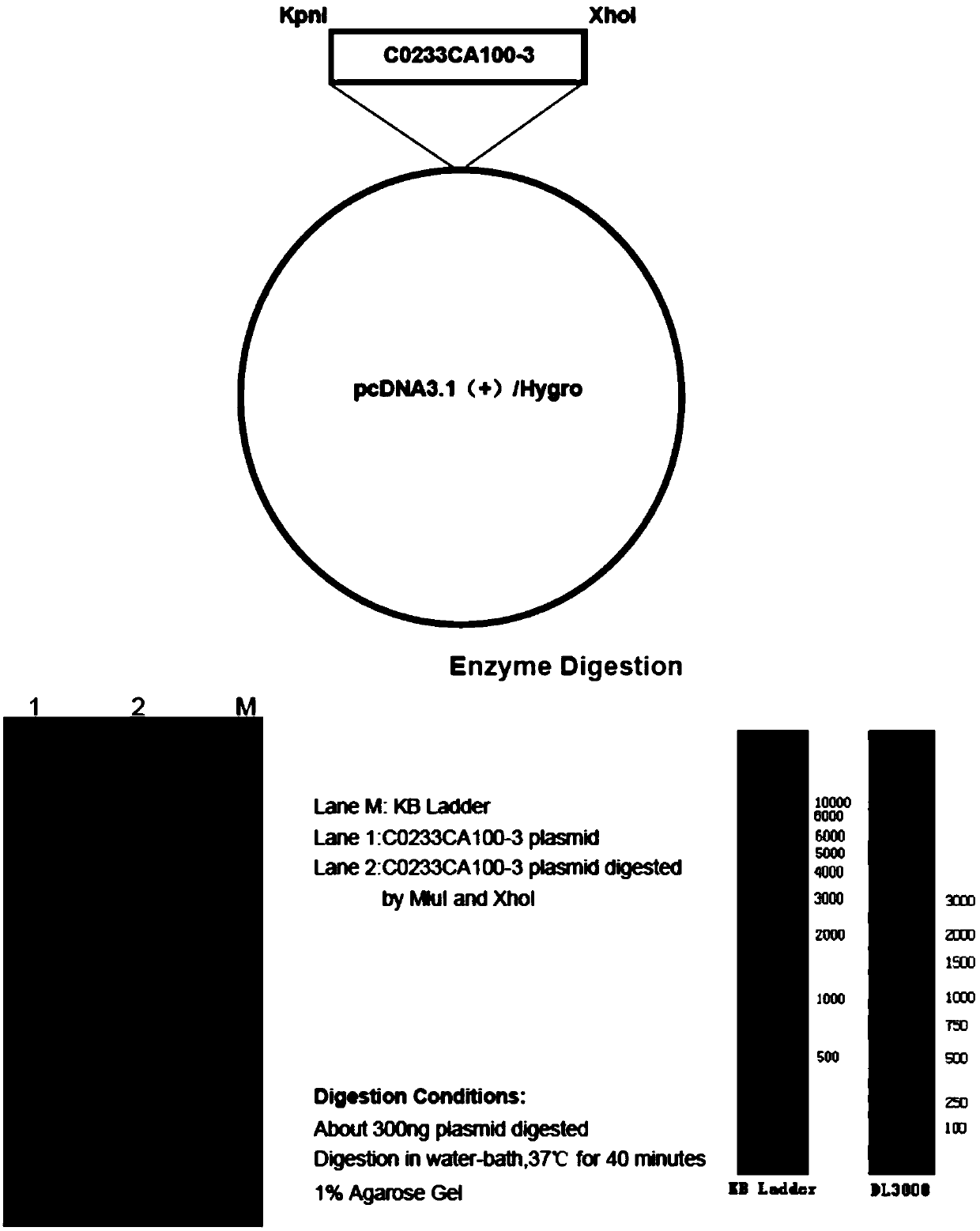
[0036] 1. 6His-NaV1.1 plasmid synthesis
[0037] A KpnI restriction site (ggtacc) was added to the 5' end of the CDS of NaV1.1, the stop codon TAA was removed from the 3' end, and a 6His tag + stop codon TAA+XhoI restriction site (catcatcatcatcatcat+taa+ctcgag) was added. His tag is added to the C-terminus of Nav1.1 protein to form the sequence shown in SEQ ID No.2.
[0038] SEQ ID No.2:
[0039] GGTACC CTCGAG
[0040] In SEQ ID No.2, 5' end GGTACC is the KpnI restriction site, For the initial password, 3' end CATCATCATCATCATCAT for the 6His tag, for the termination password, CTCGAG It is the XhoI restriction site. SEQ IDNo.2 removes the KpnI restriction site GGTACC , XhoI restrictio...
Embodiment 2、6
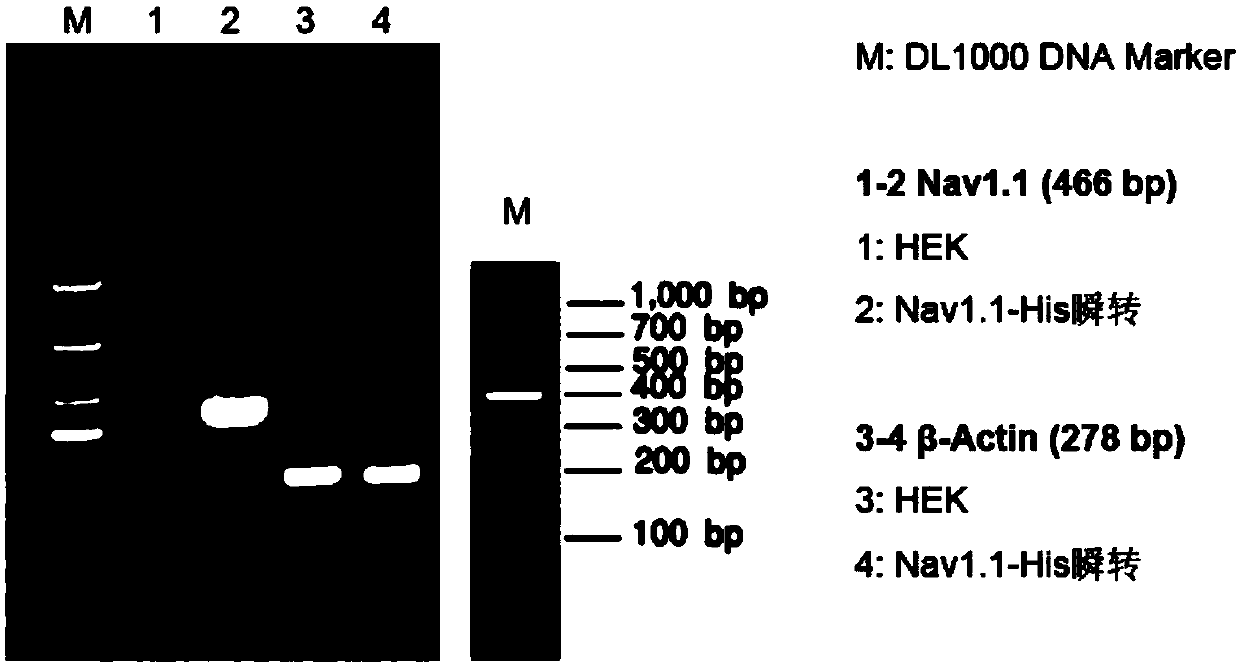
[0055] Expression of embodiment 2, 6His-Nav1.1 fusion protein
[0056] Example 1 After the cell line stably expressing the 6His-Nav1.1 fusion protein is screened out, HEK293 cells can be conventionally cultured and subcultured. which is:
[0057] The medium is: DMEM supplemented with FBS (fetal bovine serum) at a final concentration of 10%, and Hygromycin B (hygromycin B) at a final concentration of 200 μg / ml.
[0058] The culture conditions are: at 37°C, 5% CO 2 Culture in an incubator; the cells need to be subcultured when they grow to about 80%.
[0059] Subculture steps include: culture based on heating in a 37°C water bath until the medium temperature reaches or exceeds room temperature; absorb the original medium in the culture dish, wash it once with PBS, add 0.25% trypsin to digest for about 1min, add medium to stop digestion, Gently pipette the cells off the surface of the culture dish, and at the same time there are no cell clumps visible to the naked eye in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com