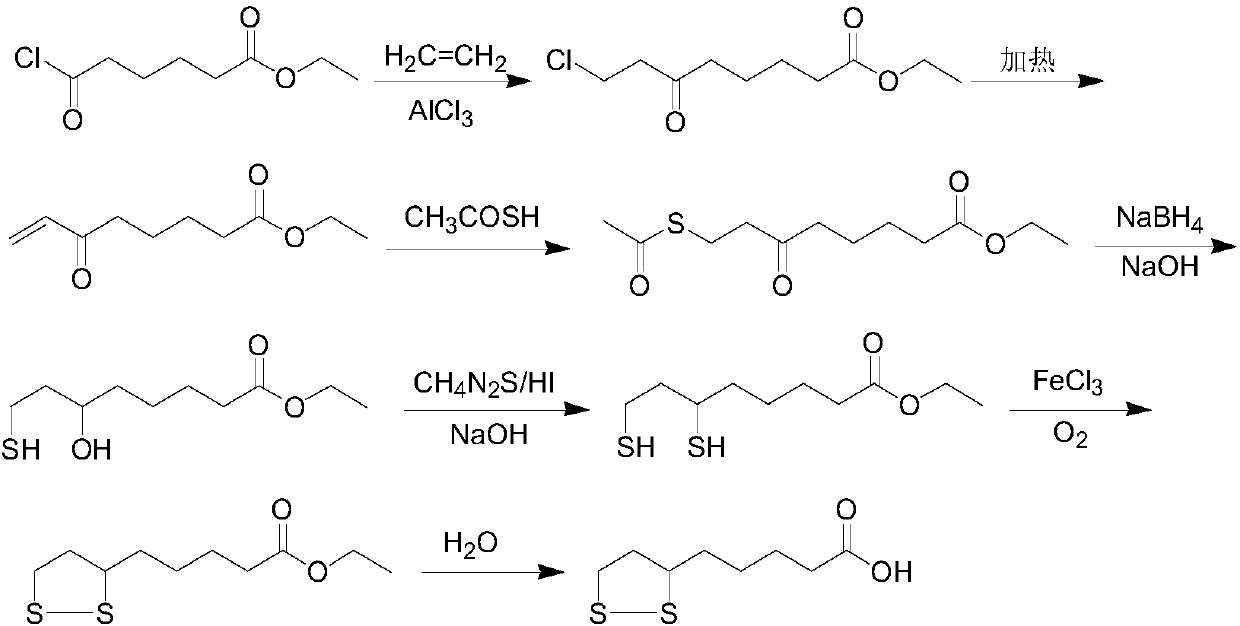
Lipoic acid preparation method and application of lipoic acid in preparation of drug for treating oligospermia
A technology of lipoic acid and lipoic acid ethyl ester, which can be used in pharmaceutical combinations, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve problems such as the meaninglessness of impurity B control, patient harm, and restricting the application of lipoic acid, and achieve easy access. Industrial production, simple method, and the effect of improving sperm motility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Into the reaction flask, 24.1 g of ethyl 6,8-dichlorooctanoate, 3.8 g of sulfur powder, 192 mL of isopropanol and 48 mL of propanol were sequentially put into the reaction flask, stirred, protected by nitrogen, protected from light, and heated to reflux. After refluxing, start to add sodium sulfide solution (30g sodium sulfide nonahydrate dissolved in a mixed solvent of 80mL water, 32mL isopropanol and 8mL propanol). The addition time is 2.5h. After the addition is complete, add the aqueous ammonium bisulfite solution (29.7g ammonium bisulfite dissolved in 75mL water), stirred at 70°C for 1h, cooled to 35°C, added 300mL water and 200mL cyclohexane, adjusted the pH of the aqueous phase to 1 with 10wt% hydrochloric acid, stirred for 15min, and separated layers , To obtain a cyclohexane solution of ethyl lipoic acid.
[0063] Add the cyclohexane solution of ethyl lipoic acid ethyl ester, 400 mL of water and 6 g of sodium hydroxide into the reaction flask, protect from light a...
Embodiment 2
[0065] Into the reaction flask, 24.1 g of ethyl 6,8-dichlorooctanoate, 6.4 g of sulfur powder, 48 mL of methanol and 48 mL of isopropanol were sequentially put into the reaction flask, stirred, protected by nitrogen, protected from light, and heated to reflux. After refluxing, start to add sodium sulfide solution (36g sodium sulfide nonahydrate dissolved in 80mL water, 20mL isopropanol and 20mL methanol), the dripping time is 1.5h, after the addition is complete, add potassium sulfite aqueous solution (31.7g potassium sulfite Dissolved in 75mL water), stirred at 80°C for 2h, then cooled to 35°C, added 300mL of water and 200mL of cyclohexane, adjusted the pH of the aqueous phase to 1 with 10% hydrochloric acid, stirred for 15min, and separated layers to obtain ethyl lipoic acid ethyl ester Cyclohexane solution.
[0066] Add a cyclohexane solution of ethyl lipoic acid ethyl ester, 800 mL of water and 16 g of sodium hydroxide into the reaction flask, protect from light and protect w...
Embodiment 3
[0069] Into the reaction flask, 24.1 g of ethyl 6,8-dichlorooctanoate, 5.1 g of sulfur powder, and 120 mL of ethanol were sequentially put into the reaction flask, stirred, protected by nitrogen, protected from light, and heated to reflux. After refluxing, start to add sodium sulfide solution (24g sodium sulfide nonahydrate dissolved in 80mL water, 40mL ethanol), the dripping time is 2h, after the addition is complete, add sodium bisulfite aqueous solution (10.4g sodium bisulfite dissolved in 75mL After stirring for 0.5h at 60℃, the temperature was lowered to 35℃, 300mL of water and 200mL of cyclohexane were added, and the pH of the aqueous phase was adjusted to 1 with 10wt% hydrochloric acid. After stirring for 15min, the layers were separated to obtain the cyclohexane of ethyl lipoic acid. Hexane solution.
[0070] Add a cyclohexane solution of ethyl lipoic acid, 100 mL of water, and 11.2 g of potassium hydroxide into the reaction flask, protect from light and protect with nitr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com