A kind of rosiglitazone nano-preparation and its preparation method and application
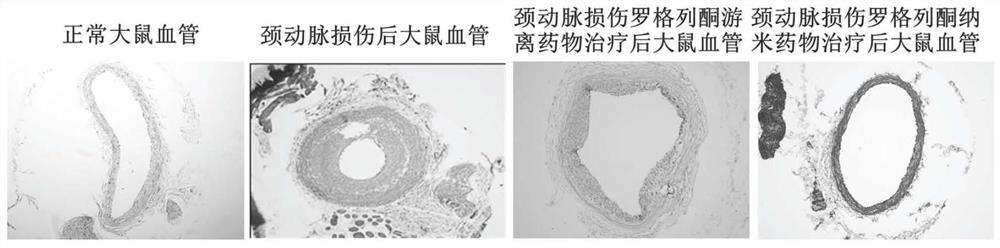
A nano-preparation, rosiglitazone technology, applied in the direction of anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve the problems of low absorption and utilization, inability to use, and short duration of biological effects, and achieve improvement Targeting, reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
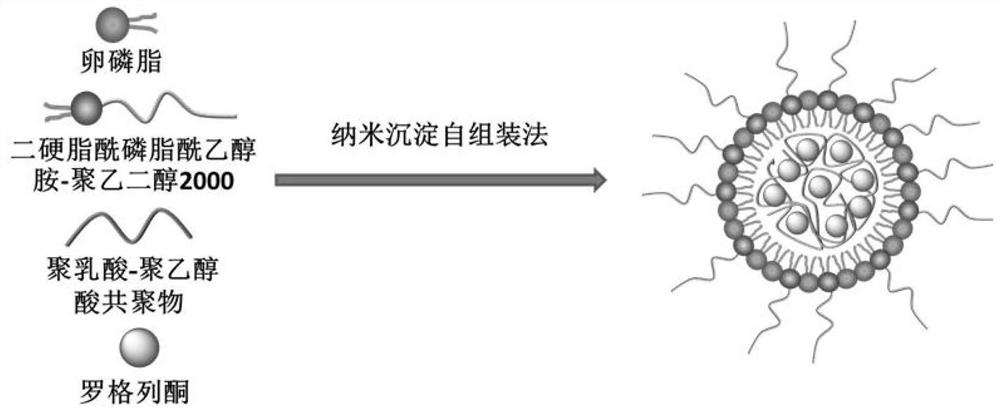
[0025] A preparation method of rosiglitazone nano-preparation, its preparation steps are as follows:
[0026] (1) dissolving rosiglitazone and polylactic acid-polyglycolic acid in an organic solvent;
[0027] (2) ultrasonically disperse lecithin and distearoylphosphatidylethanolamine-polyethylene glycol 2000 with an organic solvent, then add water to continue ultrasonically disperse, and heat at 65 degrees Celsius for 30 minutes, then cool to room temperature;
[0028] (3) Add the solution obtained in (1) dropwise to the solution in (2), stir rapidly for 3 minutes after the dropwise addition, and then continue to stir slowly at room temperature for 1.5 to 2 hours to obtain a nano-preparation suspension of rosiglitazone;
[0029] (4) centrifuging the nano-preparation suspension described in (3), washing with deionized water three times to obtain solidified rosiglitazone nano-preparation.
[0030] Calculated by mass percentage, rosiglitazone is 5.3-6.25%, polylactic acid-polygl...
Embodiment 1
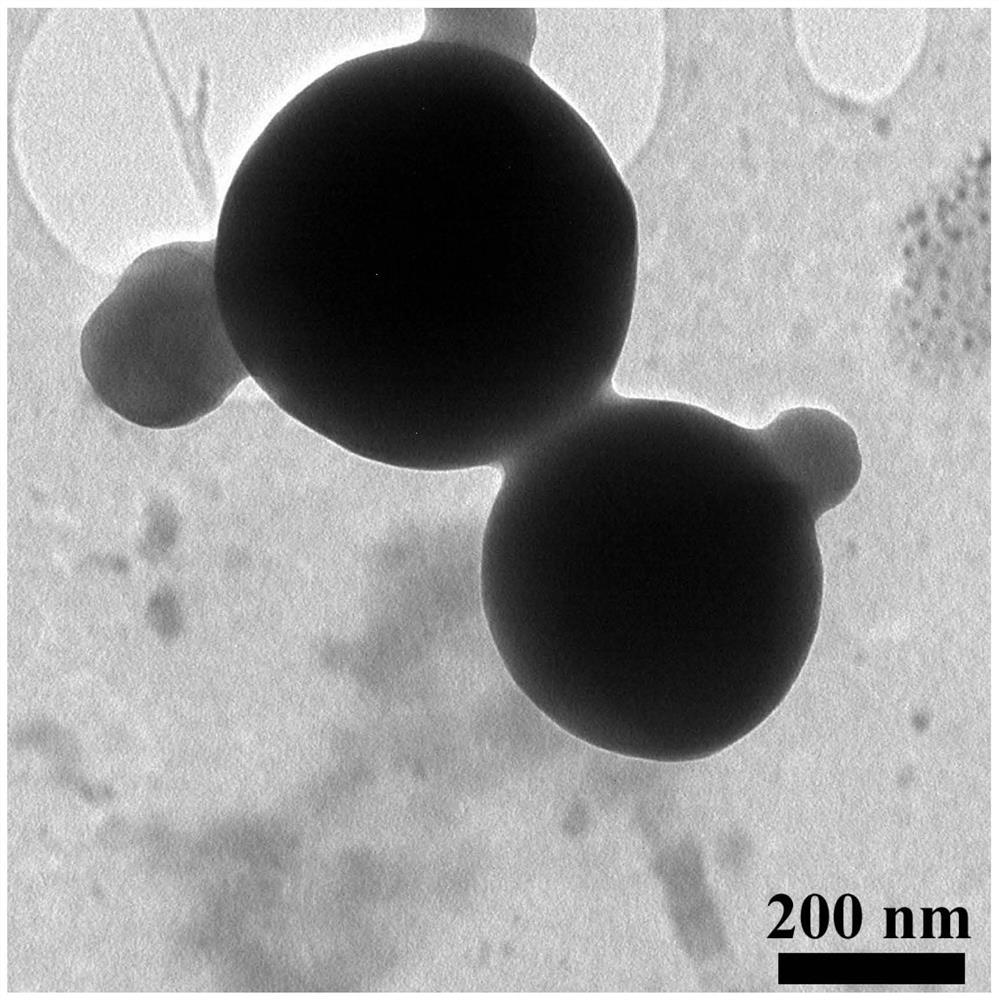
[0039] Weigh 3 mg of rosiglitazone and dissolve 30 mg of polylactic acid-polyglycolic acid in 1 ml of acetonitrile to obtain solution 1. Weigh 6 mg of lecithin and 9 mg of distearoylphosphatidylethanolamine-polyethylene glycol 2000, add them into 0.6 ml of ethanol and ultrasonically dissolve them for 3 minutes with an ultrasonic instrument. Then add 15ml of water and continue ultrasonic dissolution for 3.5 minutes. Then put it in a water bath at 65°C for 30 minutes, take it out and put it at room temperature to obtain solution 2. Add solution 1 dropwise to solution 2. After the dropwise addition, stir rapidly for 3 minutes, and then stir slowly for 1.5 hours with a magnetic stirrer. After the stirring is completed, a nano-preparation suspension of rosiglitazone is obtained. The rosiglitazone nanometer direct suspension was centrifuged at 10000 revolutions with a centrifuge, and the supernatant was discarded. Measure 45ml of deionized water, wash the precipitate three times, ...
Embodiment 2
[0041]Weigh 3 mg of rosiglitazone and dissolve 33 mg of polylactic acid-polyglycolic acid in 1.2 ml of dimethyl sulfoxide to obtain solution 1. Weigh 5 mg of lecithin and 10 mg of distearoylphosphatidylethanolamine-polyethylene glycol 2000, add them into 0.8 ml of ethanol aqueous solution, and perform ultrasonic dissolution for 3.5 minutes by an ultrasonic instrument. Then add 20ml of water and continue ultrasonic dissolution for 3 minutes. Then put it in a water bath at 65°C for 30 minutes, take it out and put it at room temperature to obtain solution 2. Add solution 1 dropwise to solution 2. After the dropwise addition, stir rapidly for 3 minutes, and then stir slowly for 2 hours with a magnetic stirrer. After the stirring is completed, a nano-preparation suspension of rosiglitazone is obtained. The rosiglitazone nanometer direct suspension was centrifuged at 10000 revolutions with a centrifuge, and the supernatant was discarded. Measure 30ml of deionized water, wash the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com