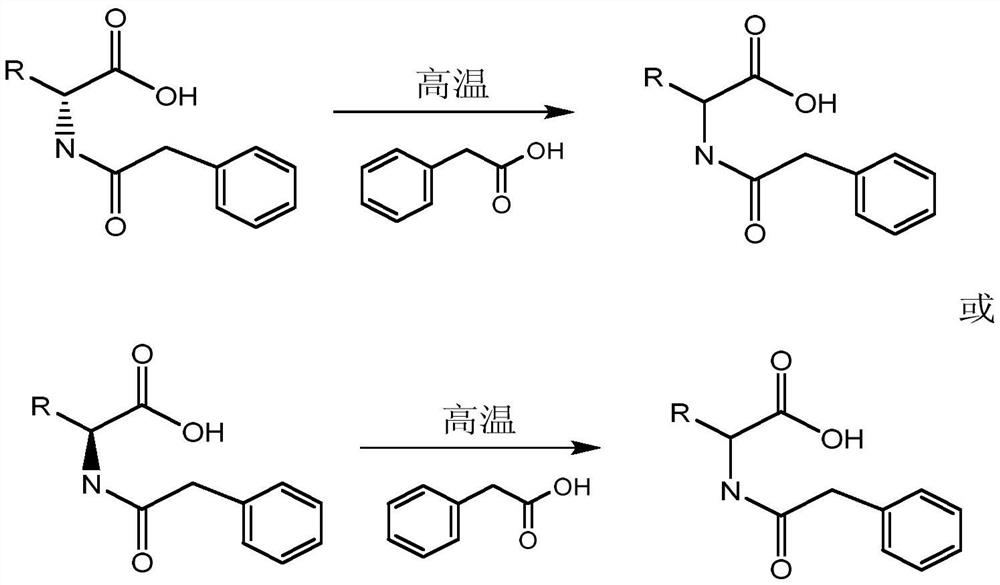
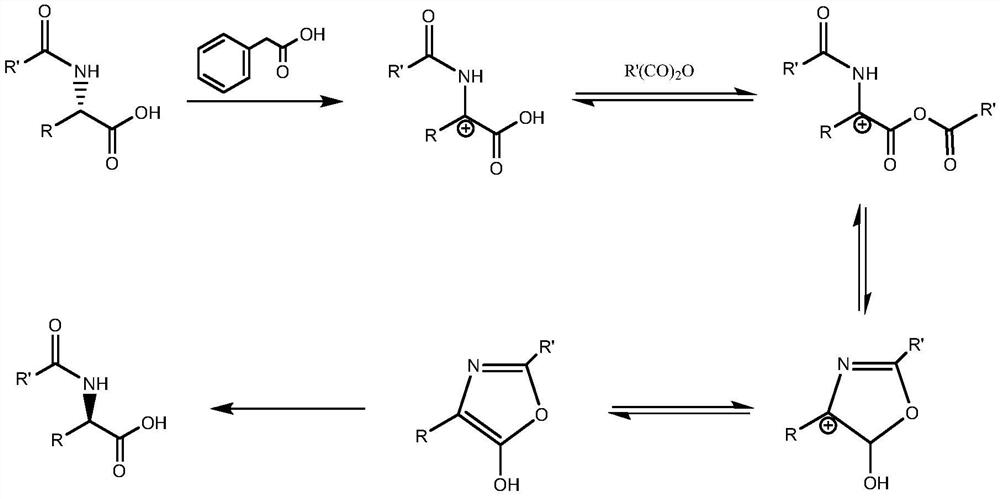
A kind of racemization method of chiral n-phenylacetyl amino acid and its derivatives
A technology for phenylacetyl and amino acids, applied in the field of racemization of amino acids and their derivatives, can solve the problems of polyacetic anhydride, easy loss of enzyme specificity, low racemization rate, etc., and achieve great economic benefits and high practical value , the effect of fast racemization speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Add 100ml of 4M NaOH solution and 19.8g of glufosinate-ammonium (0.1mol) into a 250ml three-necked flask equipped with a stirring device and stir until completely dissolved; then slowly add 18.56g (0.12mol) of benzene in an ice bath Acetyl chloride, continue to react for 4h after titration, after the reaction, separate and obtain D-N-phenylacetylglufosinate-ammonium by enzymatic resolution and subsequent treatment;
[0035] (2) Add 8.5g of phenylacetic acid (0.0625mol) to a 250ml three-necked flask, heat to a molten state and continue heating to 170°C, then add 14.9g of D-N-phenylacetylglufosinate-ammonium (0.05mol) to make the mixture rapidly reach 155 ℃, heat preservation for 5 minutes, it was in a molten state, and then cooled to 145 ℃ and kept for 15 minutes to obtain the reaction solution. After cooling the reaction solution, add ammonia water to dissolve, and then use dichloromethane to extract and remove phenylacetic acid. Finally, adjust the pH = 2 to obtain ...
Embodiment 2
[0038] (1) consistent with the method described in step (1) in Example 1;
[0039](2) Add 8.5g phenylacetic acid (0.0625mol) to a 250ml three-necked flask, heat to a molten state and continue heating to 170°C, then add 14.9g D-N-phenylacetylglufosinate-ammonium (0.05mol) and 1.0g acetic anhydride, Make the mixture quickly reach 150°C, keep it warm for 5 minutes, and turn into a molten state, then cool down to 145°C and keep it for 15 minutes to obtain the reaction solution. After the reaction solution is cooled, add ammonia water to dissolve, then use dichloromethane to extract and remove phenylacetic acid, and finally adjust the pH=2 , to obtain N-phenylacetylglufosinate-ammonium crystals.
[0040] Get a small amount of obtained N-phenylacetylglufosinate-ammonium crystals and dilute to 10g / L, and use a polarimeter to detect, and it is detected that the optical rotation is 0, that is, D-N-phenylacetylglufosinate-ammonium is completely racemized; The samples were tested before...
Embodiment 3
[0042] (1) consistent with the method described in step (1) in Example 1;
[0043] (2) Add 8.5g of phenylacetic acid (0.0625mol) to a 250ml three-necked flask, heat to a molten state and continue heating to 170°C, then add 14.9g of D-N-phenylacetylglufosinate-ammonium (0.05mol) and 3.0g of benzaldehyde, Make the mixture quickly reach 150°C, keep it warm for 5 minutes, and turn into a molten state, then cool down to 145°C and keep it for 15 minutes to obtain the reaction solution. After the reaction solution is cooled, add ammonia water to dissolve, then use dichloromethane to extract and remove phenylacetic acid, and finally adjust the pH=2 , to obtain N-phenylacetylglufosinate-ammonium crystals.
[0044] Get a small amount of obtained N-phenylacetylglufosinate-ammonium crystals and dilute to 10g / L, and use a polarimeter to detect, and it is detected that the optical rotation is 0, that is, D-N-phenylacetylglufosinate-ammonium is completely racemized; The samples were tested ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com