Preparation method of lamivudine
A technology of lamivudine and reaction time, which is applied in the direction of organic chemistry, etc., can solve the problems such as the optical purity of the product does not meet the medicinal standard, the raw materials and reagents are expensive, and are not suitable for industrial production, etc. Short, high atom utilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
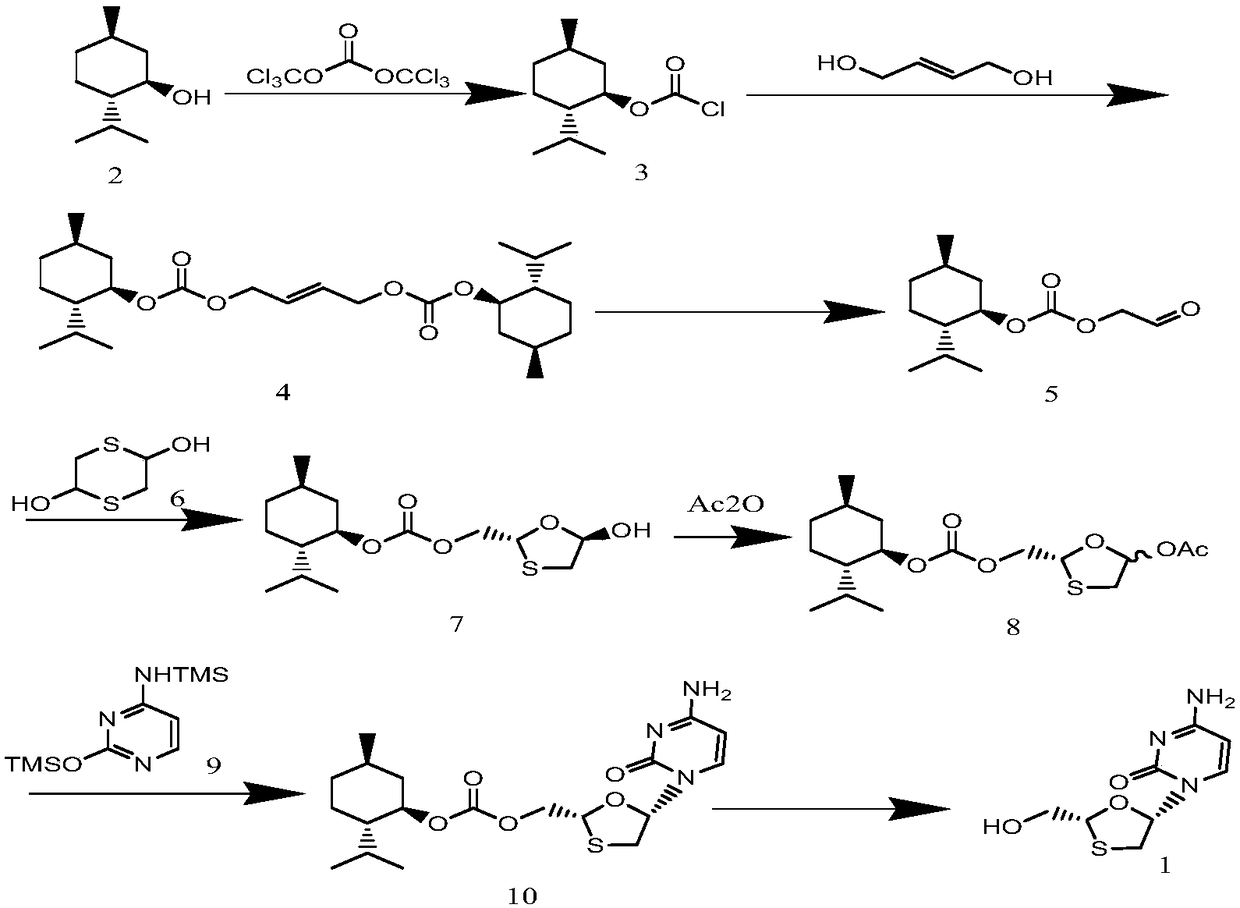
[0047] Preparation of (1R,2S,5R)-2-isopropyl-5-R-methyl-1-cyclohexyl chloroformate 2:
[0048] Add 15.60g (0.1mol) L-menthol and 156ml dichloromethane to a round-bottomed three-necked flask equipped with mechanical stirring, a thermometer, a constant-pressure feeding funnel and an exhaust gas absorption device, stir to fully dissolve, and cool to -5°C , add 14.84g (0.05mol) triphosgene and continue stirring to make it fully dissolved, then dropwise add a solution of 37.44g triethylamine and 78ml dichloromethane, and the dropwise addition is completed in about 1.5 hours. Incubate the reaction for 2 hours, enter the second reaction stage, be warming up to 25 ° C, and stir the reaction for about 6 hours. After the reaction is completed, the precipitate is separated by filtration. Washed with brine, dried with anhydrous sodium sulfate, and finally rectified under reduced pressure to collect 95-96°C, 5 mmHg fractions to obtain 19.73 g of colorless oily compound 2, with a yield of 9...
Embodiment 2
[0050] Preparation of but-2-ene-1,4-diylbis((1R,2S,5R)-2-isopropyl-5-methylcyclohexyl)bis(carbonate)4:
[0051] In a round-bottomed three-necked flask equipped with a mechanical stirring, a thermometer, and a constant-pressure feeding funnel, add 4.93g (0.052mol) butenediol, 30ml dichloromethane, stir well, cool to 0°C in an ice-water bath, add 6.48g triethyl ether amine, stir evenly, dissolve the obtained 21.88g (0.10mol) of compound 2 in 45ml of dichloromethane and slowly add it dropwise to the three-necked flask, stir and react for 1 hour, separate out the precipitate by filtration, and wash the mother liquor with saturated sodium bicarbonate solution, It was then dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain 18.98 g of compound 4 with a yield of 84%. 1 H-NMR (CDCl 3 )δ: 5.88(t, 2H), 4.77(d, 4H), 4.51(m, 2H), 1.83(m, 2H), 1.75-1.50(t, 4H), 1.63-1.38(m, 8H), 1.54 (m, 2H), 1.41 (m, 2H), 0.86 (d, 6H), 0.83 (d, 12H). Elemental Analysis C 26 O...
Embodiment 3
[0053] Preparation of (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl(2-oxoethyl)carbonate:
[0054] Add 2.52g (0.01mol) tungstic acid, 17.41ml (0.30mol) 50% H to a round-bottomed three-necked flask equipped with mechanical stirring and a thermometer in turn 2 0 2 Aqueous solution, 83ml of n-butanol solution, stirred for half an hour, then added 22.59g (0.05mol) of compound (4) obtained, stirred evenly, heated to 80°C, reacted for 5 hours, removed the catalyst by centrifugation, extracted, and the organic phase was dried with anhydrous After drying over magnesium sulfate, the solvent was distilled off under reduced pressure to obtain 18.83 g of compound 5 in a yield of 79%. 1 H-NMR (CDCl 3 ) δ: 9.65(s, 2H), 4.67(s, 2H), 4.51(m, 1H), 2.10(m, 1H), 2.00(m, 1H), 1,70(m, 2H), 1.51(m , 2H), 1.28 (m, 1H), 1.12 (m, 2H), 0.9 (m, 6H), 0.81 (d, 3H). Elemental Analysis C 13 H 22 O 4 Measured value (%): C65.48, H9.15, 026.40; Theoretical value (%) C64.44, H9.15, O26.41.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com