A kind of injection preparation containing exendin-4 Fc fusion protein
A fusion protein and injection preparation technology, applied in the field of biomedicine, can solve the problems of adverse effects, short shelf life, high production cost, etc., and achieve the effect of reducing surface interaction, ensuring quality and activity, and ensuring solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
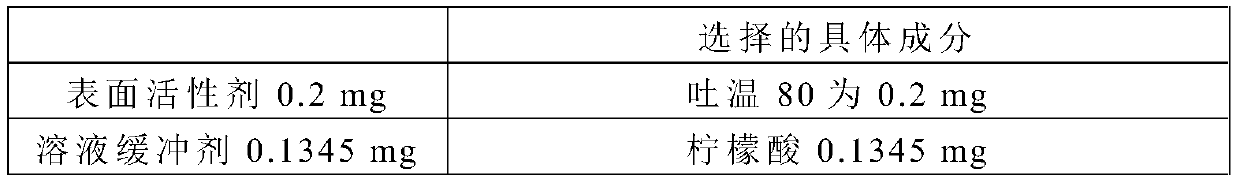
[0036] An injection preparation containing Exendin-4 Fc fusion protein, the injection preparation comprises Exendin-4 Fc fusion protein and injection mixture, the pH value of the injection mixture is 6.5, and each mL injection preparation contains 2mg Exendin-4 Fc fusion protein, The injection mixture consists of the following ingredients in grams by weight:
[0037]
[0038]
[0039] The balance is water for injection, which is added to a vial to prepare 1 mL of injection preparation.
[0040] Wherein, the amino acid sequence of the Exendin-4 Fc fusion protein is shown in SEQ ID NO.1.
Embodiment 2
[0042] An injection preparation containing Exendin-4 Fc fusion protein, the injection preparation includes Exendin-4 Fc fusion protein and injection mixture, the pH value of the injection mixture is 6.0, and each mL injection preparation contains 1.5mg Exendin-4 Fc fusion protein , the injection mixture consists of the following ingredients in grams by weight:
[0043] Select specific ingredients Surfactant 0.1mg Tween 40: 0.04mg, Poloxamer 0.06mg Solution buffer 0.1mg Phosphoric acid: 0.05mg, succinic acid: 0.05mg Osmotic regulator 5mg Fructose 2.5mg, sodium chloride 2.5mg Stabilizer 50mg Trehalose 10mg, Mannitol 10mg, Chitosan 30mg
[0044] The balance is water for injection, which is added to a vial to prepare 1 mL of injection preparation.
[0045] Wherein, the amino acid sequence of the Exendin-4 Fc fusion protein is shown in SEQ ID NO.1.
Embodiment 3
[0047] An injection preparation containing Exendin-4 Fc fusion protein, the injection preparation includes Exendin-4 Fc fusion protein and injection mixture, the pH value of the injection mixture is 6.7, and each mL injection preparation contains 2.5mg Exendin-4 Fc fusion protein , the injection mixture consists of the following ingredients in grams by weight:
[0048] Specific ingredients Surfactant 2mg Glycerin Fatty Acid Ester 1.5mg, Poloxamer 0.5mg Solution buffer 0.8mg Citric acid 0.4mg, phosphoric acid 0.4mg Osmotic regulator 10mg Sodium chloride 5mg, glucose 5mg Stabilizer 90mg Trehalose 10mg, Mannitol 50mg, Chitosan 30mg
[0049] The balance is water for injection, which is added to a vial to prepare 1 mL of injection preparation.
[0050] Wherein, the amino acid sequence of the Exendin-4 Fc fusion protein is shown in SEQ ID NO.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com