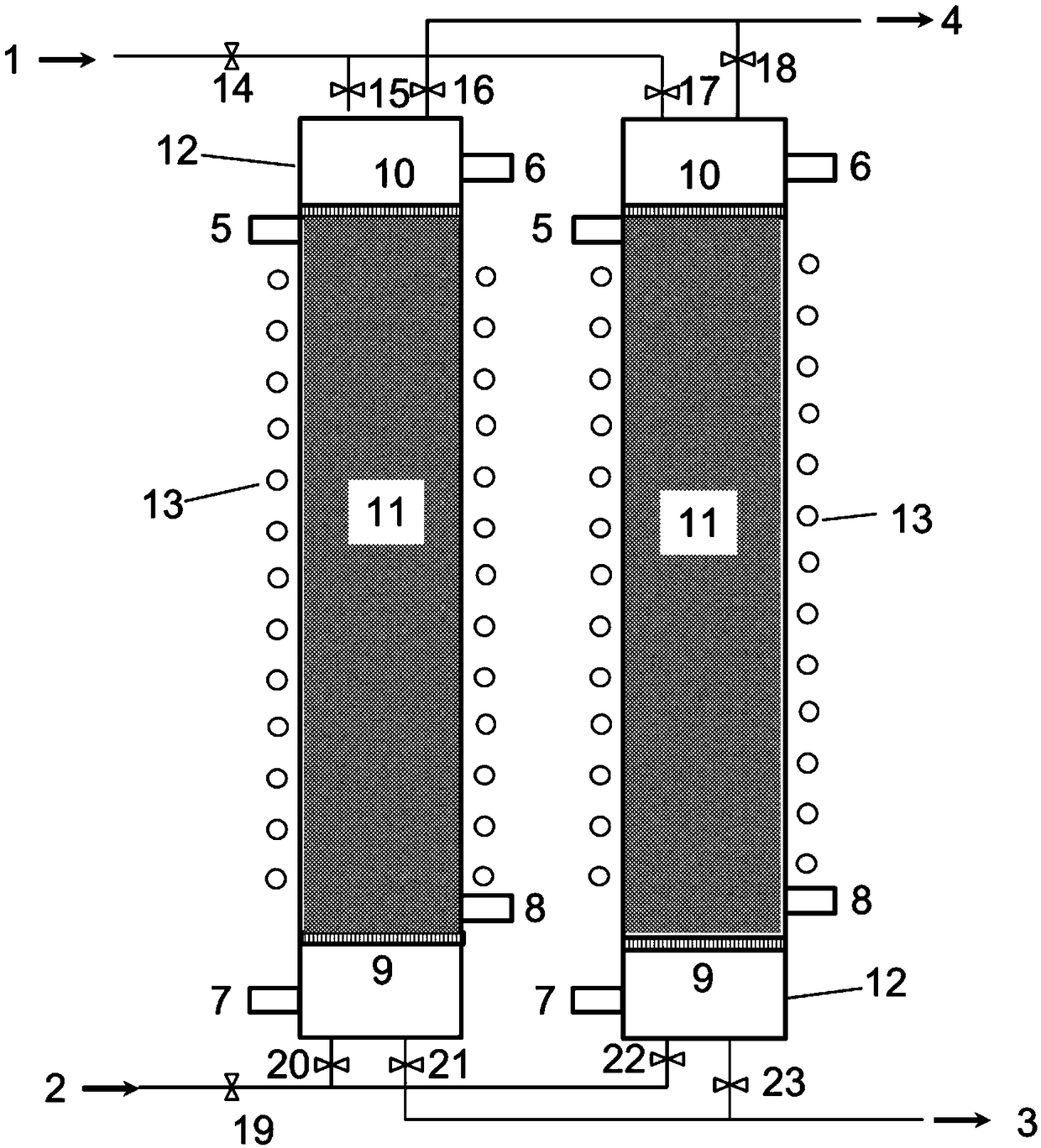
Macroporous resin and ion exchange device for adsorbing high-concentration rhenium
An ion exchange device and ion exchange column technology, applied in the ion exchange, ion exchange, anion exchange and other directions of chelate compounds, can solve the problems of low exchange capacity and efficiency, and achieve improved efficiency, increased feed concentration, and cross-linking. low degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]Adopt the method for suspension polymerization to prepare macroporous divinylbenzene-methyl acrylate copolymer skeleton, and add the pore-forming agent that is made up of isobutanol and toluene by volume ratio of 1:1; Then, adopt iron trichloride as catalyst, adopt chlorine Chloromethylation reaction was carried out between diethyl ether and macroporous divinylbenzene-methyl acrylate copolymer backbone to obtain chlorine spheres with a chlorine content of 20%. Take 100 g of the prepared chlorine spheres, soak them in methanol to swell, add methanol to a volume that is 3 times the volume of the chlorine spheres, and the swelling time is 2 h; Kylation reaction, the reaction temperature is 60 ℃, the reaction time is 10h, to obtain macroporous ion exchange resin.
[0036] The prepared macroporous resin was pretreated, then sulfated and transformed, and the transformed resin was used for adsorption experiments. Control the feed rhenium concentration to 500 mg L -1 , the tem...
Embodiment 2
[0038] Adopt the method for suspension polymerization to prepare macroporous divinylbenzene-methyl acrylate copolymer skeleton, and add the pore-forming agent that is made up of isobutanol and toluene by volume ratio of 1:1; Then, adopt iron trichloride as catalyst, adopt chlorine Chloromethylation reaction was carried out between ether and macroporous divinylbenzene-methyl acrylate copolymer backbone to obtain chlorine spheres with a chlorine content of 22%. Take 100 g of the prepared chlorine spheres, soak them in methanol to swell, add methanol to 4 times the volume of the chlorine spheres, and the swelling time is 5 h; Kylation reaction, the reaction temperature is 70 ℃, the reaction time is 10h, to obtain macroporous ion exchange resin.
[0039] The prepared macroporous resin was pretreated, then sulfated and transformed, and the transformed resin was used for adsorption experiments. Control the feed rhenium concentration to 500 mg L -1 , the temperature is 25 ℃, the fl...
Embodiment 3
[0041] Adopt the method for suspension polymerization to prepare macroporous divinylbenzene-methyl acrylate copolymer skeleton, and add the pore-forming agent that is made up of isobutanol and toluene by volume ratio of 1:1; Then, adopt iron trichloride as catalyst, adopt chlorine The chloromethylation reaction between diethyl ether and macroporous divinylbenzene-methyl acrylate copolymer backbone resulted in chlorine spheres with a chlorine content of 21%. Take 100 g of the prepared chlorine spheres, soak them in methanol to swell, add methanol to a volume 3 times the volume of the chlorine spheres, and the swelling time is 10 h; Kylation reaction, the reaction temperature is 80 ℃, the reaction time is 15h, to obtain macroporous ion exchange resin.
[0042] The prepared macroporous resin was pretreated, then sulfated and transformed, and the transformed resin was used for adsorption experiments. Control the feed rhenium concentration to 500 mg L -1 , the temperature is 25 ℃...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| aspect ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com