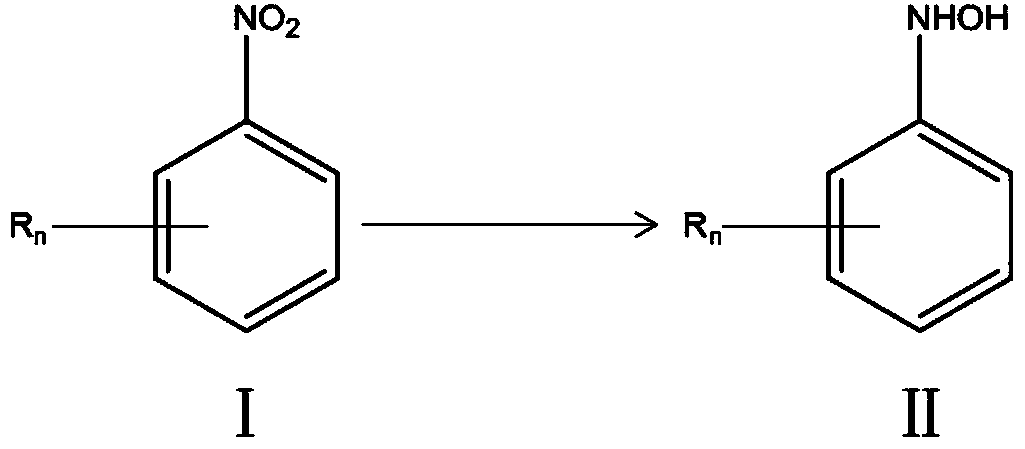
Catalytic hydrogenation catalyst as well as preparation thereof and application thereof to selective hydrogenation reaction of aromatic nitro compound
A catalytic hydrogenation and catalyst technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve the problem of reducing catalyst activity and service life, easily poisoning catalyst, Complicated post-treatment process and other issues, to avoid steric hindrance effect, good catalytic performance, and improve metal utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
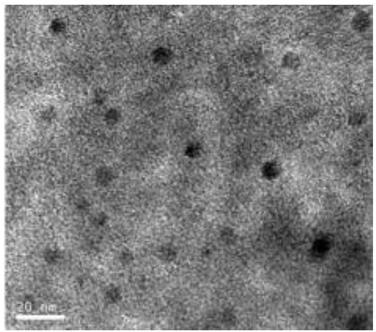
[0036] Weigh 1.5g of methyl orthosilicate and 0.05g of n-butyl titanate and add them to the distillation flask, add 20g of activated carbon after fully stirring, and then add dropwise an aqueous solution of tetramethylammonium hydroxide (containing tetramethylammonium hydroxide) with a mass concentration of 20%. Hydroxyl ammonium hydroxide 0.075g), distill and separate the hydrolyzate at 95°C, and then heat the solid matter in the flask at 180°C for 12h to obtain activated carbon coated with a modified coating.
[0037] Get the gac (70% of mesopore ratio after detection, 30% of micropore ratio, size is not more than 1cm) 1g and 15ml of deionized water and 3ml of methyl alcohol that are coated with modified coating that the above-mentioned method makes and make mixed slurry, stir 15min, take 10mL palladium concentration of 0.002g / mL chloropalladium acid solution dropwise into the mixed solution, put it under the ultraviolet lamp, and carry out light stirring for 40min under the ...
Embodiment 14
[0048] The catalyst prepared in Example 1 was loaded into a tubular reactor with an inner diameter of 20 cm, and the air was replaced with nitrogen, and then the nitrogen was replaced with hydrogen. According to the total volume of the reaction solution composed of p-chloronitrobenzene and methanol to the volume ratio of hydrogen is 1:1, the ratio of p-chloronitrobenzene to methanol is 2g:5ml, and the p-chloronitrobenzene is dissolved in methanol solvent to obtain the reaction The liquid is pumped into the mixing tube in front of the reaction tube by a high-pressure liquid pump, mixed with hydrogen, and then enters the tubular reactor loaded with catalyst. The hydrogen pressure is set at 1MPa, the temperature is 50°C, and the liquid space velocity is 8min. -1 , turn on the lighting device, set the light power to 325nm, the power to 225W, and the radiation intensity to 3820μW / cm 2 , start the reaction, pass through the gas-liquid separation device after the one-way reaction, th...
Embodiment 15~27
[0050] Examples 15-27 are based on the fixed-bed catalytic evaluation operation steps in Example 14. The fixed-bed catalytic hydrogenation catalysts prepared in Examples 1-13 and Comparative Examples 1-2 are selected for catalyst reaction. The specific parameters are shown in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com