Synthesis and application of p-methoxyl-containing Schiff base type visible light photosensitizer with conjugated structure
A technology of conjugated structure and p-methoxy group is used in the synthesis and application of visible light photosensitizers, which can solve the problems of poor solubility of dyes and need to improve the initiation efficiency, and achieves convenient and easy-to-obtain raw material sources and large molar extinction. Coefficients, Methods of Combining and Separating Simple Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The synthesis of 4-(4-(4-methoxybenzylamino) styryl)-N,N-dimethylaniline is carried out in three steps:
[0040] (1) N,N-Dimethyl-4-(4-nitrostyryl)aniline
[0041] Put 2.733g of p-nitrophenylacetic acid and 1.500g of p-dimethylaminobenzaldehyde into a three-necked flask with a molar ratio of 1.5:1 and mix them, add a condensing device, and add 1.271g (about 1.5ml) dropwise under constant stirring. For hydropyridine, reflux at 100°C for 3 hours, then raise the temperature to 130°C and continue to reflux for 4-5 hours until no bubbles come out and stop the reaction. The solid obtained after the reaction was recrystallized twice with absolute ethanol, and dried in a vacuum oven for further use, with a yield of 95%.
[0042] (2) Synthesis of 4-(4-aminostyryl)-N,N-dimethylaniline
[0043] Add 0.750 g of the first step product N, N-dimethyl-4-(4-nitrostyryl) aniline and 1.263 g of stannous chloride dihydrate into a three-necked flask in a molar ratio of 1:2 and mix , under...
Embodiment 2
[0047] The synthesis of N, N-diethyl-4-(4-(4-methoxybenzylamino) styryl) aniline is carried out in three steps:
[0048] (1) 4-(4-nitrostyryl)-N,N-diethylaniline
[0049] Add 1.470g of p-nitrophenylacetic acid and 1.500g of p-4-(diethylamino)benzaldehyde in a three-necked flask with a molar ratio of 1.5:1 and mix them. Add a condensing device and add 1.271g (approx. 1.5ml) hexahydropyridine, reflux at 100°C for 3 hours, then raise the temperature to 130°C and continue to reflux for 4 to 5 hours until no bubbles emerge to stop the reaction. The dark red solid obtained after the reaction was recrystallized twice with absolute ethanol, and dried in a vacuum oven for further use, with a yield of 94%.
[0050] (2) 4-N, the synthesis of N-diethyl-(4-aminostyryl) aniline
[0051] Add 1.500 g of the first step product 4-(4-nitrostyryl)-N,N-diethylaniline and 2.045 g of stannous chloride dihydrate into a three-necked flask in a molar ratio of 1:2 and mix , under continuous stirring,...
Embodiment 3
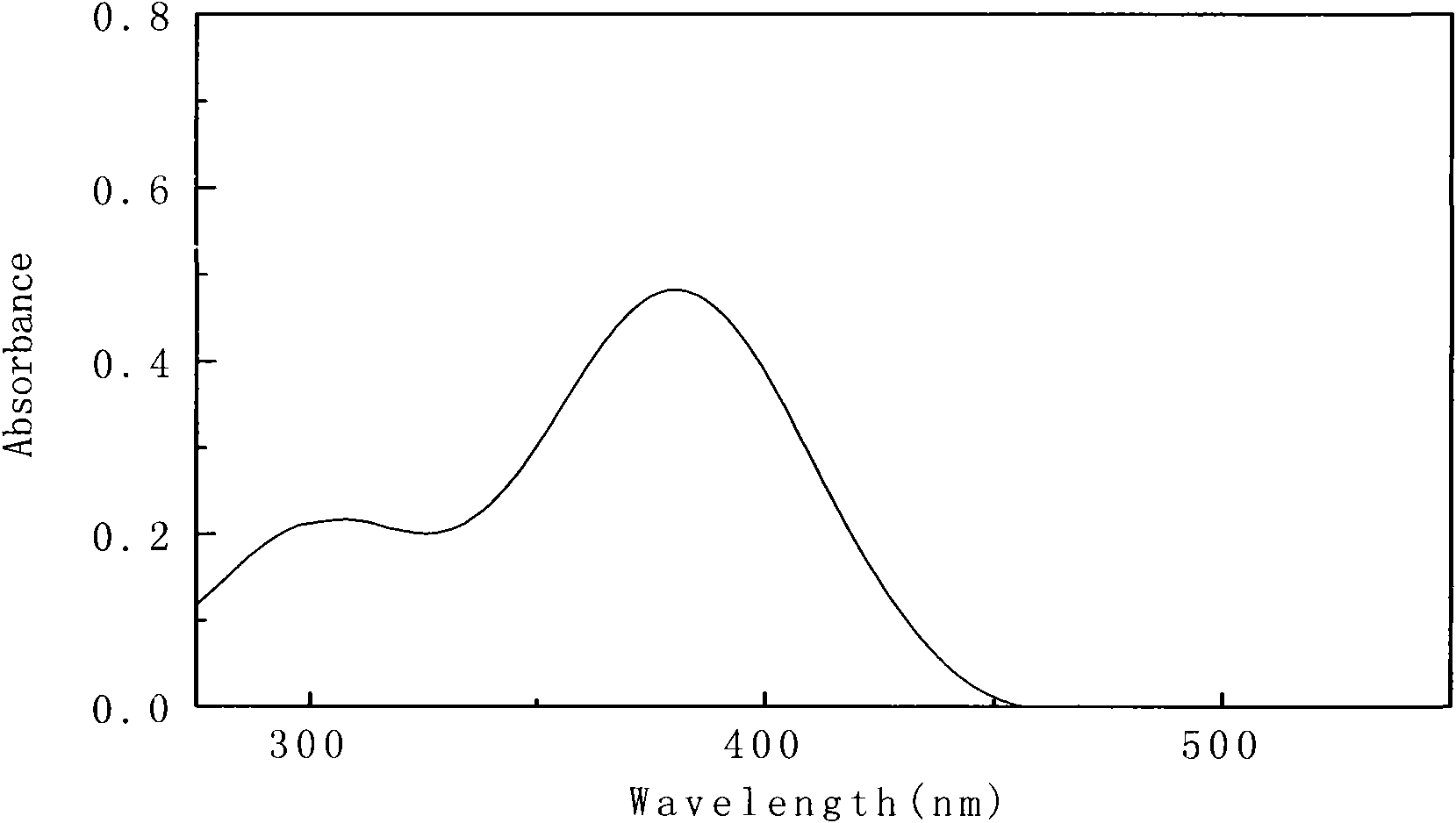
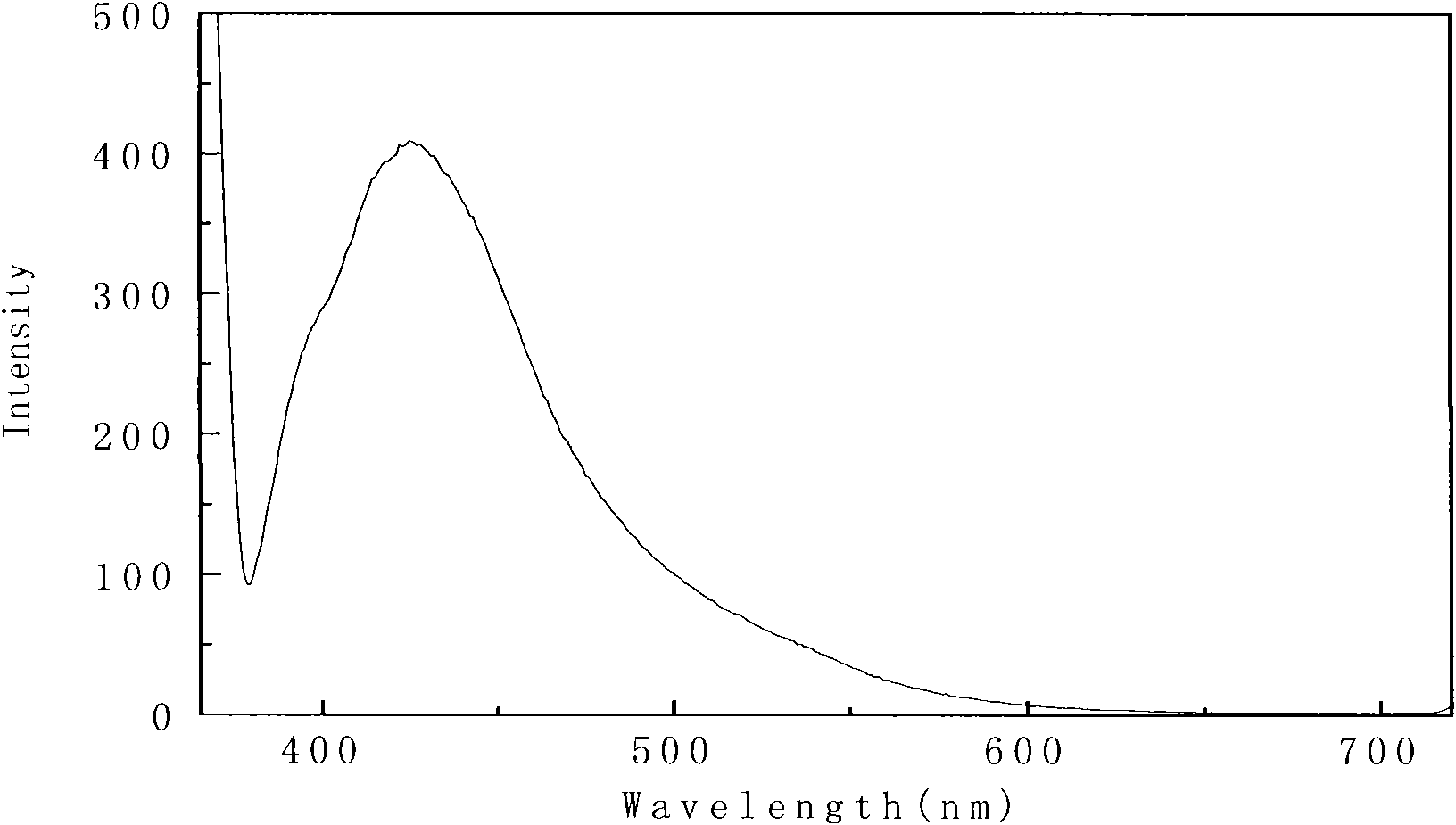
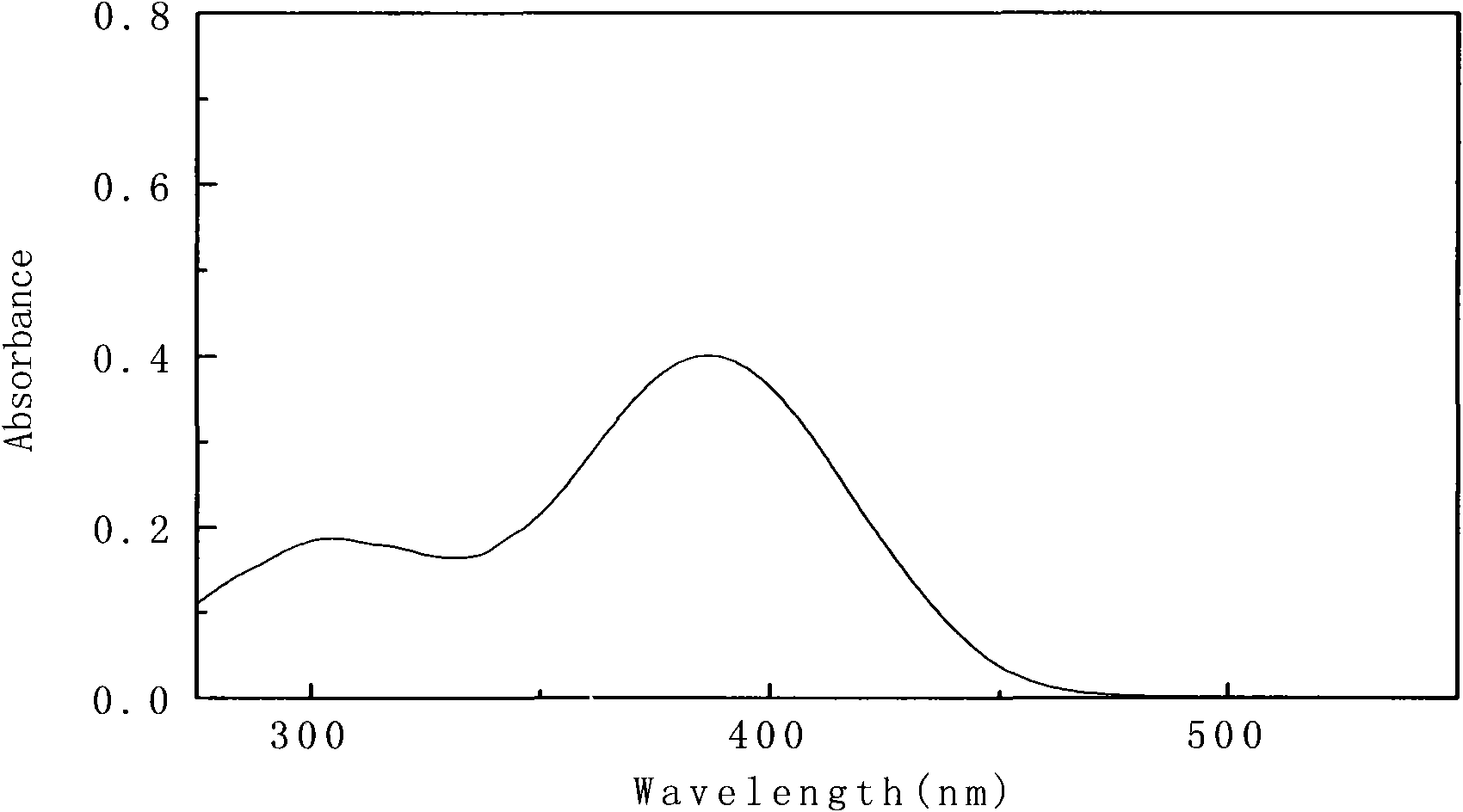
[0055] Will 1×10 -5 The 4-(4-(4-methoxybenzylamino) styryl)-N,N-dimethylaniline of mol / L is dissolved in ethyl acetate, and its ultraviolet-visible absorption spectrum is measured, as figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com