Kit applied to detecting apoA I and apo B and preparation method of stabilizer of kit
A kit and stabilizer technology, which is applied in the field of medical testing, can solve problems such as poor anti-interference ability, large differences between bottles, and different fixed values of coal belts, and achieve the effect of prolonging storage life and service life and enhancing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
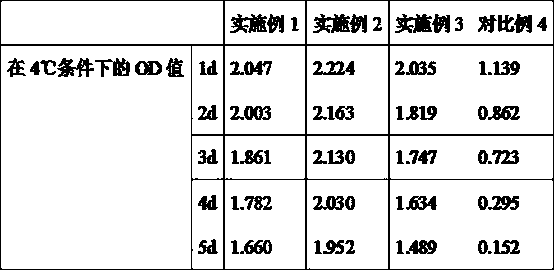
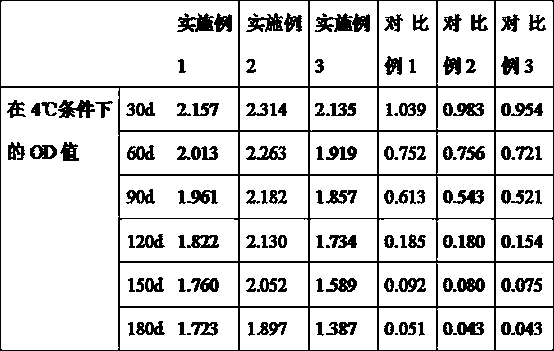
[0023] Embodiment 1: A kind of kit for detecting apoA I, apoB, comprising apoA antibody liquid, apoB antibody liquid and liquid serotype constant value calibration liquid, also includes stabilizer;
[0024] According to parts by weight, the stabilizer includes 1 part of trehalose, 100 parts of 0.01 mol / L phosphate buffer solution, 1 part of horseradish peroxidase, 0.01 part of sodium propionate and 1 part of thimerosal.
[0025] The above-mentioned apoA antibody solution, apoB antibody solution and liquid serotype constant value calibration solution were all prepared according to the scheme mentioned in the Chinese patent authorization announcement number CN101135687B.
[0026] Modification of trehalose;
[0027] Step1: Put a beaker containing 25ml of 1mol / L sodium hydroxide on a stirrer and stir at a speed of 1500r / min, add 5.7g of trehalose, and slowly add 1.3g of carboxymethyl cellulose under rapid stirring system, then add 4g of 1,4-butanediol diglycidyl ether into the be...
Embodiment 2
[0034] Embodiment 2: A kind of kit for detecting apoA I, apoB, comprising apoA antibody liquid, apoB antibody liquid and liquid serotype constant value calibration liquid, also includes stabilizer;
[0035] According to parts by weight, the stabilizer includes 3 parts of trehalose, 150 parts of 0.01mol / L phosphate buffer solution, 3 parts of horseradish peroxidase, 0.05 parts of sodium propionate and 3 parts of thimerosal.
[0036] The above-mentioned apoA antibody solution, apoB antibody solution and liquid serotype constant value calibration solution were all prepared according to the scheme mentioned in the Chinese patent authorization announcement number CN101135687B.
[0037] Modification of trehalose;
[0038] Step1: Put a beaker containing 25ml of 1mol / L sodium hydroxide on a stirrer and stir at a speed of 1500r / min, add 5.7g of trehalose, and slowly add 1.3g of carboxymethylcellulose under rapid stirring system, then add 4g of 1,4-butanediol diglycidyl ether into the ...
Embodiment 3
[0045] Embodiment 3: a kind of kit for detecting apoA I, apoB, including apoA antibody liquid, apoB antibody liquid and liquid serotype constant value calibration liquid, also includes stabilizer;
[0046] According to parts by weight, the stabilizer includes 5 parts of trehalose, 200 parts of 0.01mol / L phosphate buffer solution, 5 parts of horseradish peroxidase, 0.1 part of sodium propionate and 5 parts of thimerosal.
[0047] The above-mentioned apoA antibody solution, apoB antibody solution and liquid serotype constant value calibration solution were all prepared according to the scheme mentioned in the Chinese patent authorization announcement number CN101135687B.
[0048] Modification of trehalose;
[0049] Step1: Put a beaker containing 25ml of 1mol / L sodium hydroxide on a stirrer and stir at a speed of 1500r / min, add 5.7g of trehalose, and slowly add 1.3g of carboxymethylcellulose under rapid stirring system, then add 4g of 1,4-butanediol diglycidyl ether into the bea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com