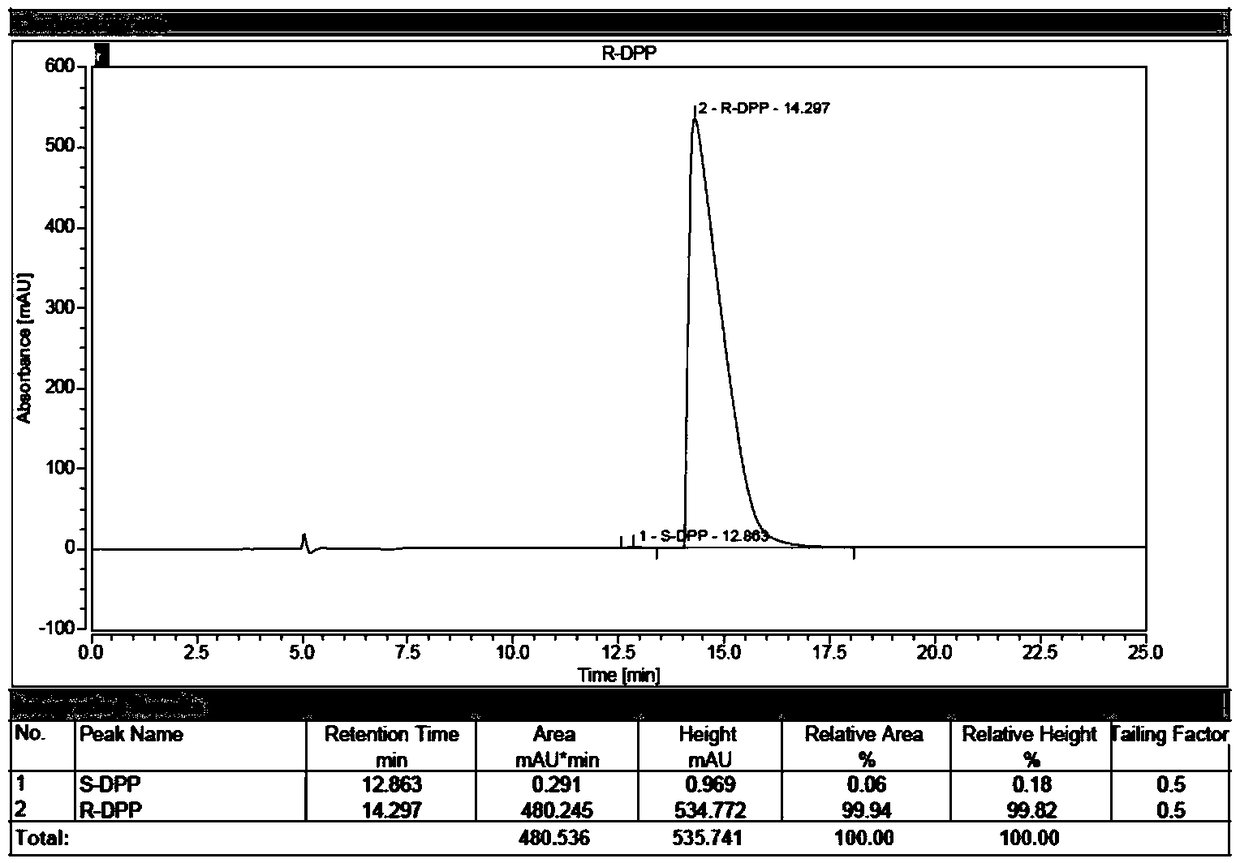
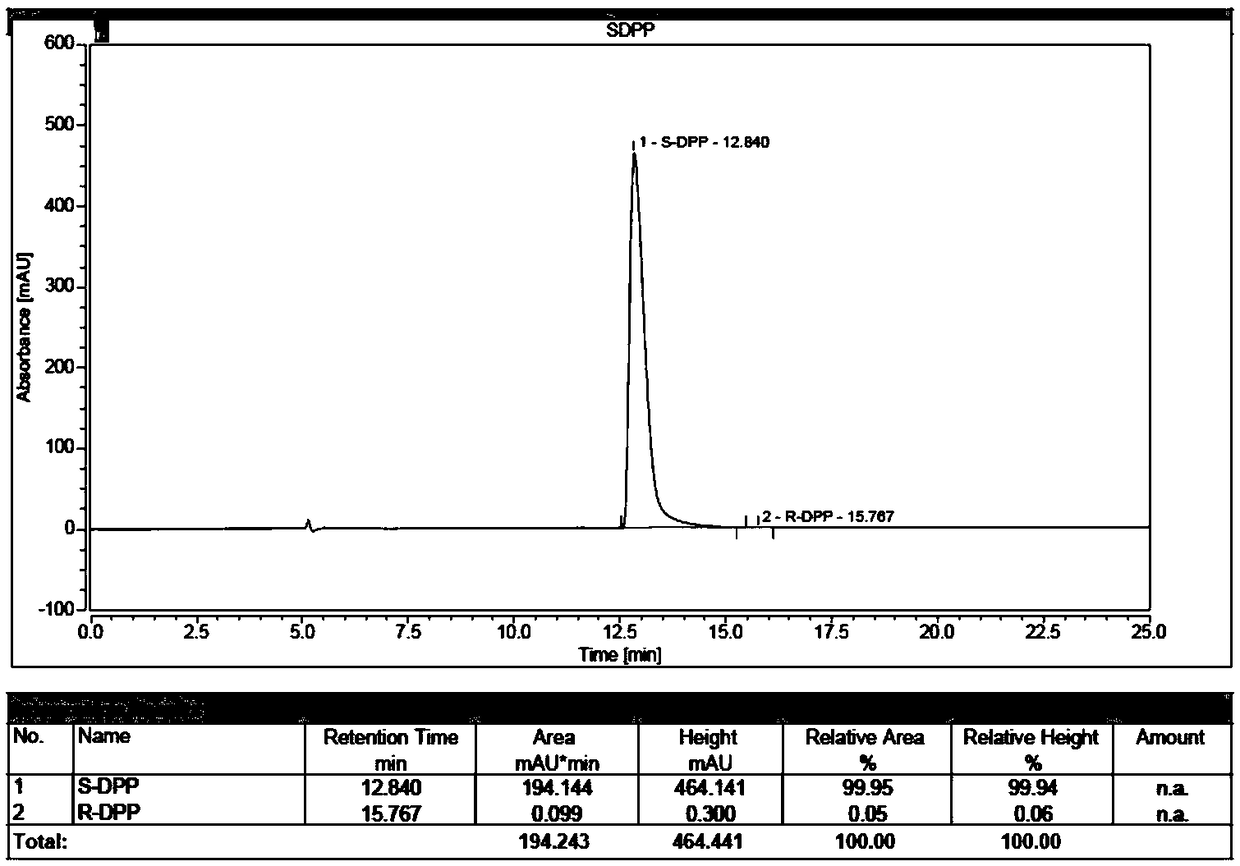
Synthesis process of chiral catalyst
A chiral catalyst and synthesis process technology, applied in organic chemistry, organic chemistry methods, bulk chemical production, etc., can solve the problems of complex production process, high irritation, high control of low temperature energy consumption, etc., to meet the needs of large-scale production Need, mild reaction conditions, controllable reaction temperature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The industrialized production of embodiment 1 (R)-diphenylprolinol hydrochloride
[0078]
[0079] 1. A 100-liter reactor was pumped into 22.0 kilograms of anhydrous methanol under nitrogen protection. Control the internal temperature of the reactor below 30°C. 7.0 kg of concentrated sulfuric acid (mass concentration 98%) is slowly pumped into the reactor. Open the lid of the reaction kettle, and quickly add 8.0 kg of D-proline. After the addition, adjust the inner temperature of the reaction kettle to 25-30° C., and continue stirring for 7 hours. The solvent was distilled off under reduced pressure at 55°C.
[0080] 2. Pump 35.0 kg of dichloromethane into the reactor. Cool down to 15°C, add 14.8 kg of sodium carbonate in batches, keep the temperature below 20°C during the addition, and continue stirring for 30 minutes after the addition. Pump the dichloromethane (11.2 kg) solution of di-tert-butyl dicarbonate (16.1 kg) into the supporting dropping tank of the re...
Embodiment 2
[0085] The industrialized production of embodiment 2 (R)-diphenylprolinol hydrochloride
[0086] 1. A 100-liter reactor was pumped into 22.0 kilograms of anhydrous methanol under nitrogen protection. Control the internal temperature of the reactor below 30°C. Slowly pump 7.0 kg of concentrated sulfuric acid into the reactor. Open the lid of the reaction kettle, and quickly add 8.0 kg of D-proline. After the addition, adjust the inner temperature of the reaction kettle to 25-30° C., and continue stirring for 7 hours. The solvent was distilled off under reduced pressure at 55°C.
[0087] 2. 34.3 kg of tetrahydrofuran was pumped into the reaction kettle. Cool down to 15°C, add 19.2 kg of potassium carbonate in batches, keep the temperature below 20°C during the addition, and continue stirring for 30 minutes after the addition. Pump the dichloromethane (11.2 kg) solution of di-tert-butyl dicarbonate (16.1 kg) into the supporting dropping tank of the reaction kettle, add dropwi...
Embodiment 3
[0090] The industrialized production of embodiment 3 (S)-diphenylprolinol hydrochloride
[0091]
[0092] 1. A 100-liter reactor was pumped into 22.0 kilograms of anhydrous methanol under nitrogen protection. Control the internal temperature of the reactor below 30°C. Slowly pump 7.0 kg of concentrated sulfuric acid into the reactor. Open the lid of the reaction kettle, and quickly add 8.0 kg of L-proline. After the addition, adjust the inner temperature of the reaction kettle to 25-30° C., and continue stirring for 7 hours. The solvent was distilled off under reduced pressure at 55°C.
[0093] 2. Pump 35.0 kg of dichloromethane into the reactor. Cool down to 15°C, add 14.8 kg of sodium carbonate in batches, keep the temperature below 20°C during the addition, and continue stirring for 30 minutes after the addition. Pump the dichloromethane (11.2 kg) solution of di-tert-butyl dicarbonate (16.1 kg) into the supporting dropping tank of the reaction kettle, add dropwise at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com