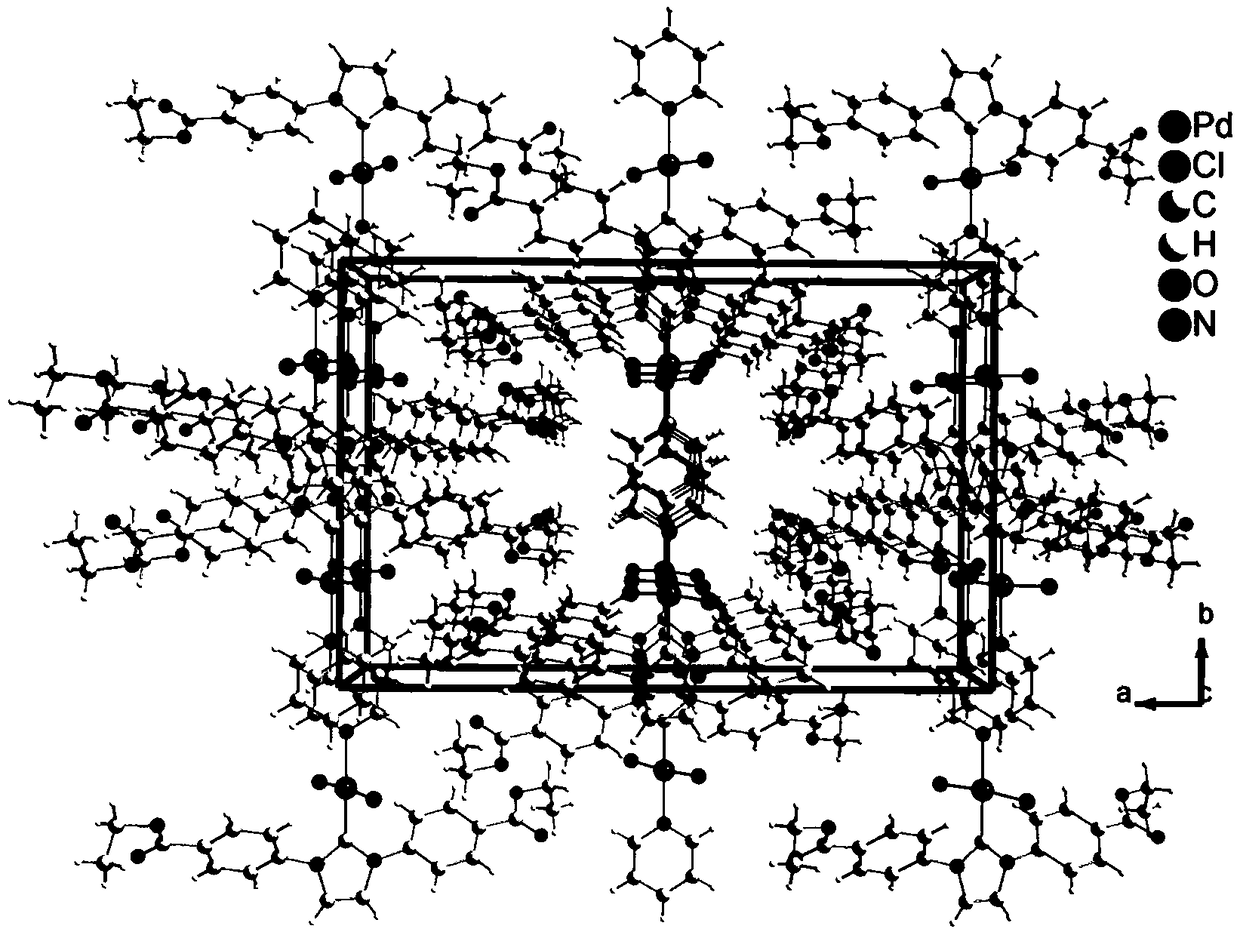
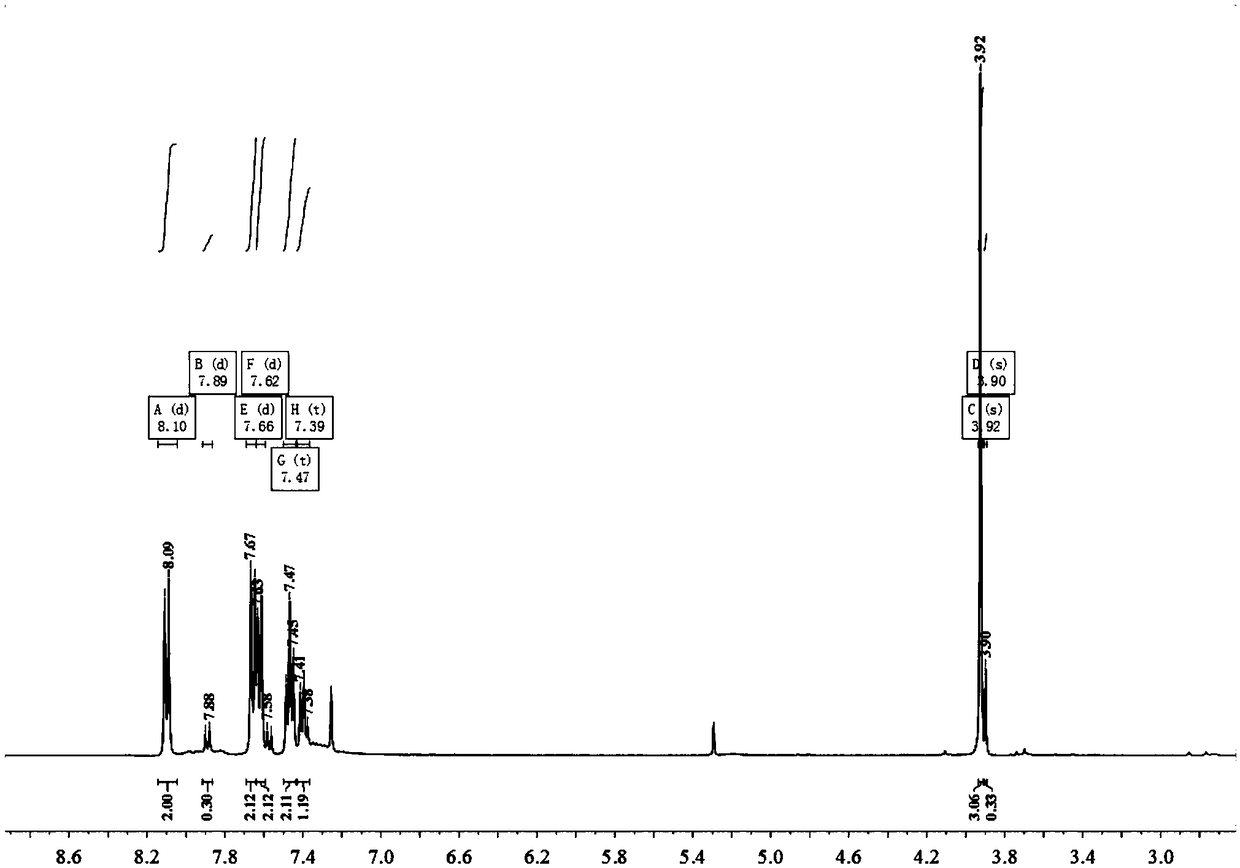
Crystal structure of ester Pd-NHC molecular complex and synthetic method
A synthesis method and complex technology, which are used in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, organic chemistry methods, etc. Electronic structure and other issues, to achieve the effect of good catalytic activity and excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
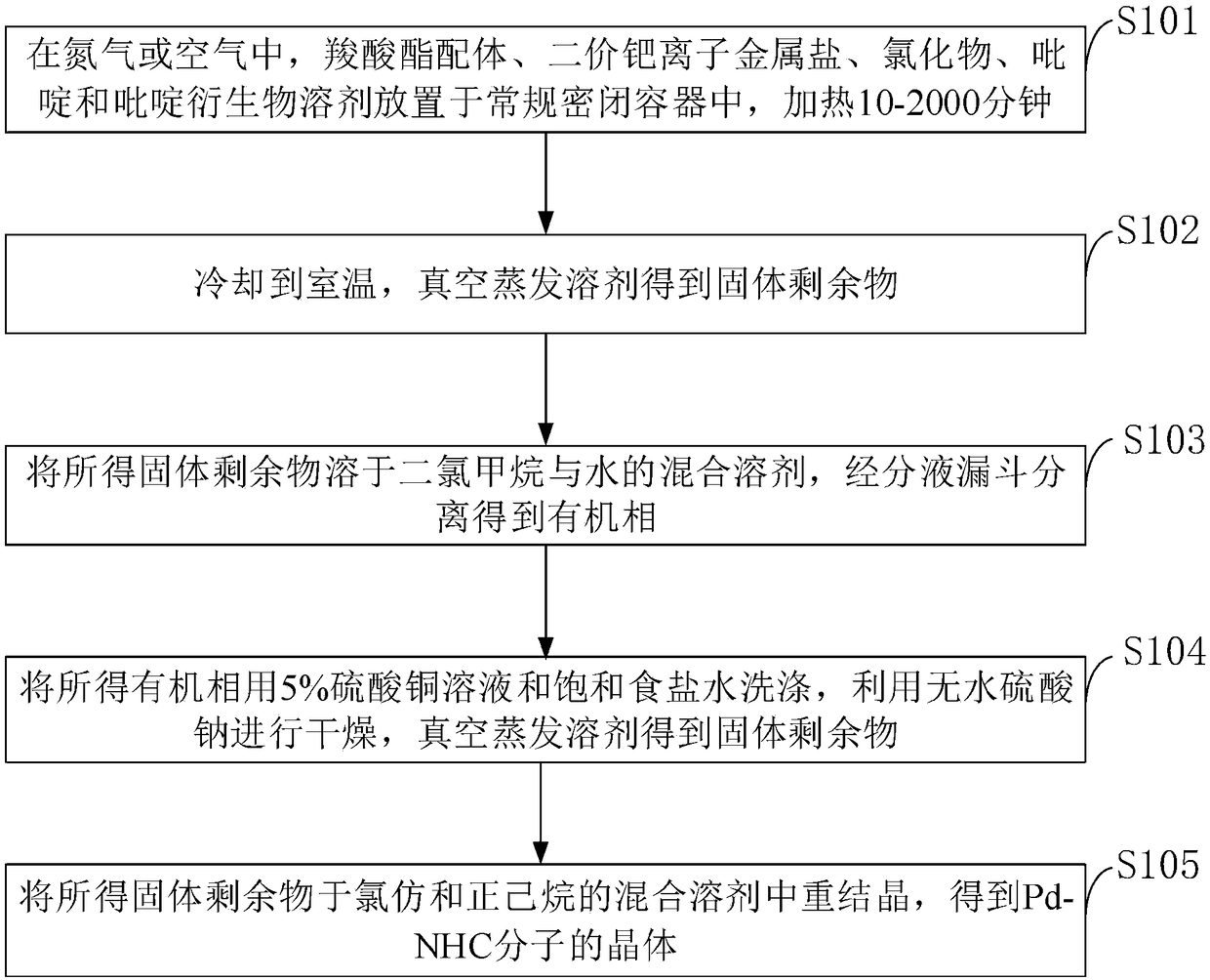
[0047] Such as figure 1 As shown, the synthesis method of the ester Pd-NHC molecular complex provided by the embodiment of the present invention specifically includes the following steps:
[0048] S101: In nitrogen atmosphere or air, carboxylate ligand, divalent palladium ion metal salt (0.05-1.0mmol), chloride, pyridine and pyridine derivative solvent are placed in a conventional airtight container, and heated for 10-2000 minutes;
[0049] S102: cooling to room temperature, evaporating the solvent in vacuo to obtain a solid residue;
[0050]S103: dissolving the obtained solid residue in a mixed solvent of dichloromethane and water, and separating through a separatory funnel to obtain an organic phase;
[0051] S104: washing the obtained organic phase with 5% copper sulfate solution and saturated brine, drying with anhydrous sodium sulfate, and evaporating the solvent in vacuo to obtain a solid residue;
[0052] S105: Recrystallize the obtained solid residue in a mixed solv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com