Method of preparing 3-amino-2-thiocyano-alpha, beta-unsaturated compound
A technology for thiocyano group and compound, which is applied in the field of visible light-driven synthesis of 3-amino-2-thiocyano-α,β-unsaturated compounds, can solve the problems of low yield, by-products, toxicity, etc. The effect of high utilization rate, lower industrial cost and abundant sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of 3-amino-2-thiocyanobutenoic acid ethyl ester
[0026]
[0027] At room temperature, ethyl acetoacetate (65.1mg, 0.5mmol), ammonium thiocyanate (114, 2mg, 1.5mmol), fluorescein (3.3mg, 2mol%) were added to a 10mL reaction tube, and then the solvent acetonitrile was added 2mL, under the irradiation of 3.0W blue LED, react in the air for 6 hours, and detect by TLC. After the reaction was completed, the reaction mixture was concentrated in vacuo, and the remaining crude product was separated by column chromatography to obtain 88.4 mg of ethyl 3-amino-2-thiocyanobutenoate as a white solid, with a yield of 95%.
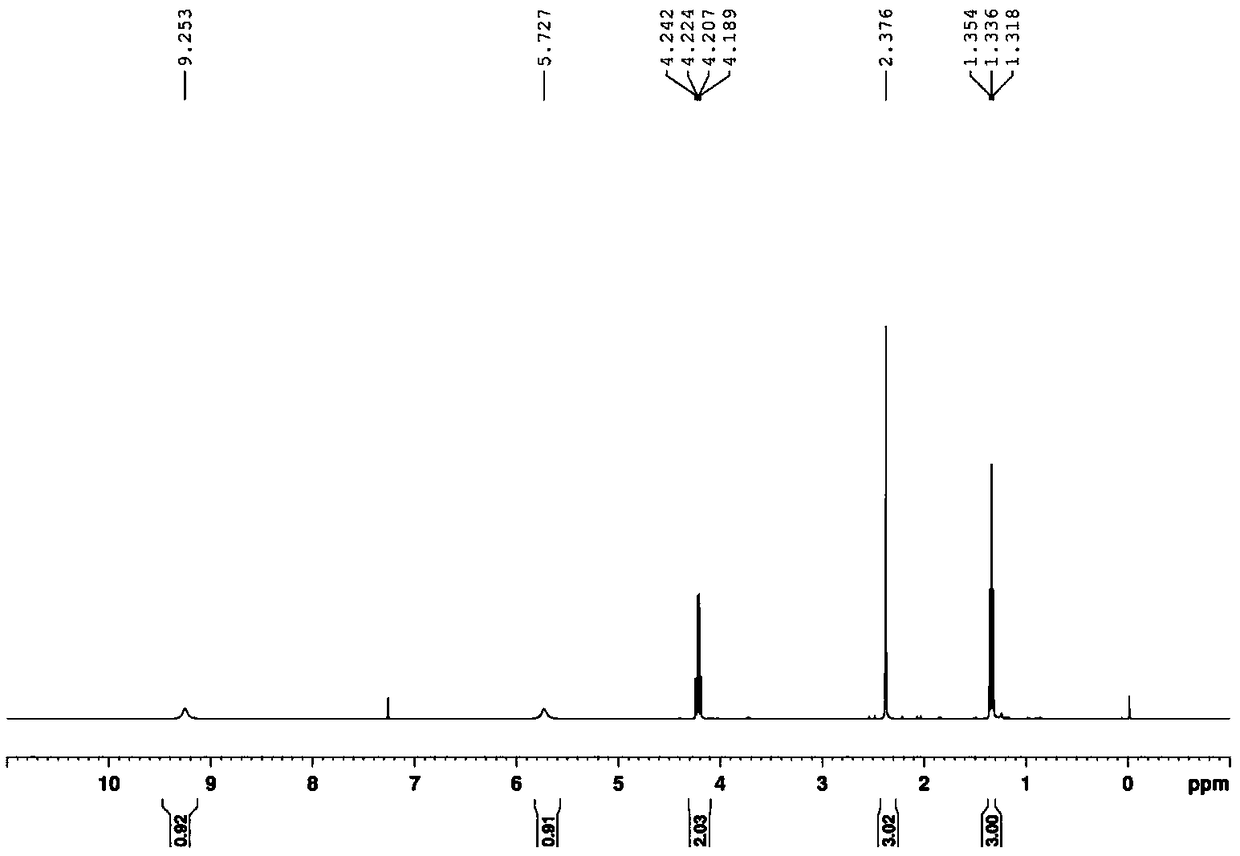
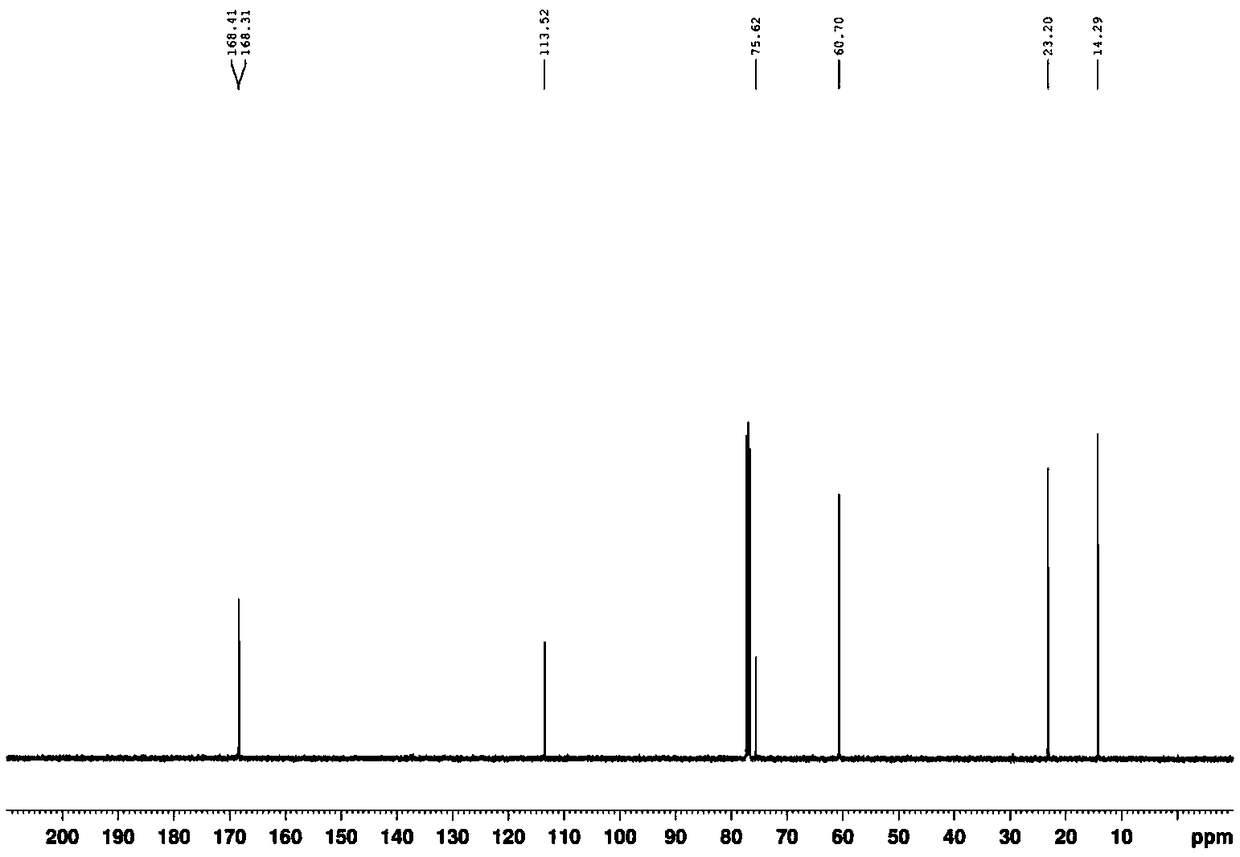
[0028] Ethyl(E)-3-amino-2-thiocyanatobut-2-enoate White solid, (95% yield) 1 HNMR (400MHz, CDCl 3 )δ9.25(s, 1H), 5.73(s, 1H), 4.22(q, J=7.1Hz, 2H), 2.38(s, 3H), 1.34(t, J=7.1Hz, 3H); 13 C NMR (100MHz, CDCl 3 )δ168.4, 168.3, 113.5, 75.6, 60.7, 23.2, 14.3; MS (ESI, m / z): Calculated for [C 7 h 10 N 2 o 2 S](M+H) + 187.0, found 187.0. ...
Embodiment 2
[0029] Example 2: Synthesis of 3-amino-2-thiocyanobutenoic acid methyl ester
[0030]
[0031] At room temperature, methyl acetoacetate (58 mg, 0.5 mmol), ammonium thiocyanate (114, 2 mg, 1.5 mmol), and fluorescein (3.3 mg, 2 mol%) were added to a 10 mL reaction tube, and then 2 mL of solvent acetonitrile was added , reacted in the air for 6 hours under the irradiation of 3.0W blue LED, and detected by TLC. After the reaction was completed, the reaction mixture was concentrated in vacuo, and the remaining crude product was separated by column chromatography to obtain 79.1 mg of 3-amino-2-thiocyanobutenoic acid methyl ester as a white solid, with a yield of 92%.
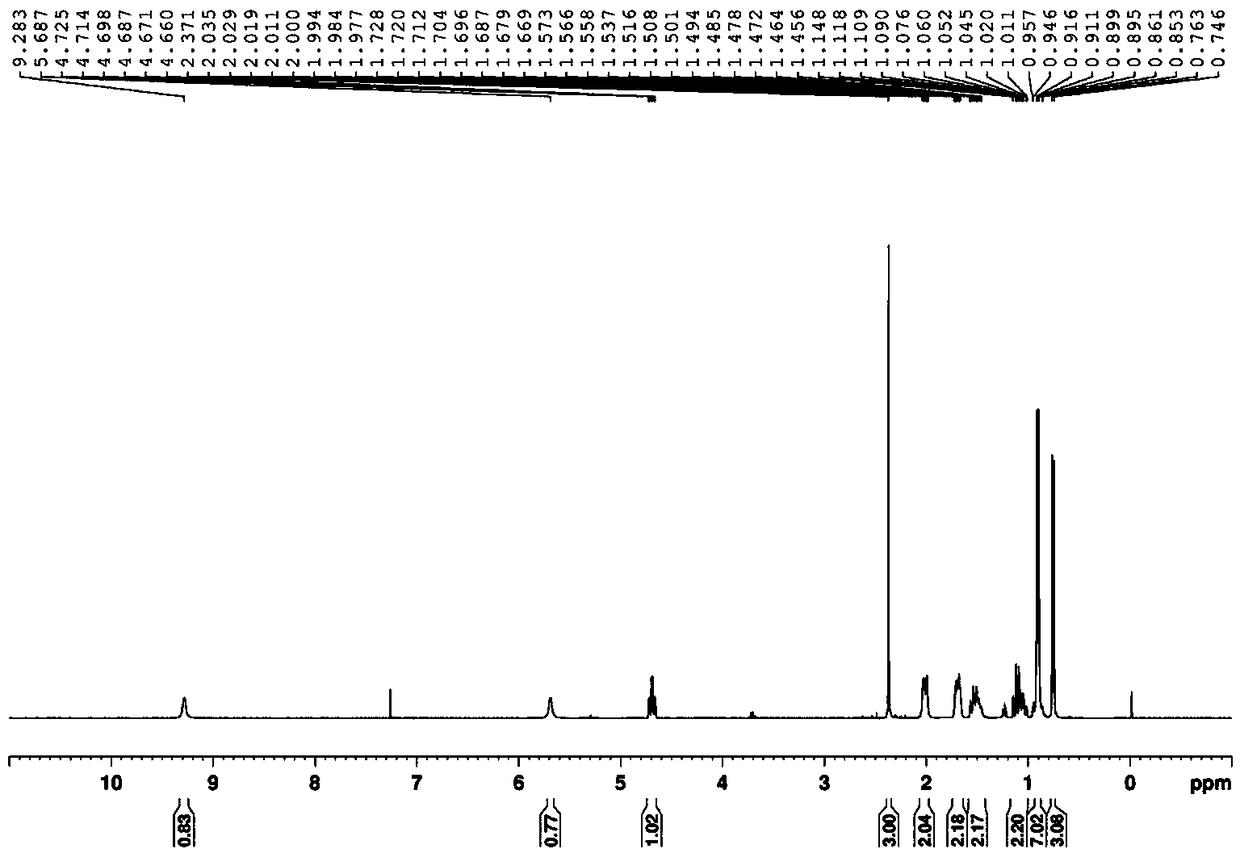
[0032] Methyl(E)-3-amino-2-thiocyanatobut-2-enoate White solid, (92% yield); 1 H NMR (400MHz, CDCl 3 )δ9.24(s,1H),5.82(s,1H),3.77(s,3H),2.38(s,3H); 13 C NMR (100MHz, CDCl 3 )δ168.7, 168.6, 113.4, 75.5, 51.9, 23.2; MS (ESI, m / z): Calculated for [C 6 h 8 N 2 o 2 S](M+H) + 173.0, found 173.0.
Embodiment 3
[0033] Example 3: Synthesis of 3-amino-2-thiocyanobutenoic acid isopropyl ester
[0034]
[0035] At room temperature, isopropyl acetoacetate (72.1mg, 0.5mmol), ammonium thiocyanate (114,2mg, 1.5mmol), fluorescein (3.3mg, 2mol%) was added to a 10mL reaction tube, and then the solvent was added 2mL of acetonitrile was reacted in air for 6 hours under the irradiation of 3.0W blue LED, and detected by TLC. After the reaction was completed, the reaction mixture was concentrated in vacuo, and the remaining crude product was separated by column chromatography to obtain 90 mg of isopropyl 3-amino-2-thiocyanobutenoate as a white solid, with a yield of 90%.
[0036] Isopropyl(E)-3-amino-2-thiocyanatobut-2-enoate White solid, (90% yield); 1 H NMR (CDCl 3 ,400MHz)δ9.23(s,1H),5.85(s,1H),5.06-4.96(m,1H),2.35(s,3H),1.30(s,3H),1.29(s,3H); 13 C NMR (CDCl 3,100MHz) δ168.2,167.9,113.7,75.8,68.1,23.2,21.9; HRMS(ESI):m / z calculated for C 8 h 12 N 2 o 2 S[M+H] + 201.0692, found 201.069...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com