A kind of preparation method of polysubstituted benzoate
A benzoate and multi-substituted technology is applied in the field of preparation of multi-substituted benzoates, and can solve the problems of low yield, cumbersome synthesis route, difficult post-processing and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
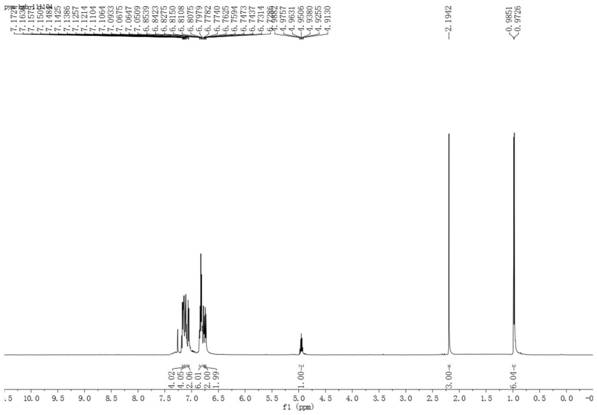
[0029] Add 3mL of toluene to a 10mL round bottom flask, then add 1mmol ethyl acetoacetate and 1mmol toluene and mix uniformly to obtain a mixed solution. Add 5% cuprous chloride of the total mass of the mixed solution as a catalyst to form a reaction system. The reaction system was heated to 90 degrees Celsius, and the reaction was stirred for 16 hours under the condition that the temperature of the reaction system was kept at 90 degrees Celsius. After the reaction, the reaction product obtained was extracted three times with ethyl acetate and water 30mL (volume ratio 2:1), and a certain amount of anhydrous magnesium sulfate was added to the obtained upper organic phase to stand overnight for drying, and the solvent toluene was evaporated under reduced pressure. The crude product was obtained, and the crude product was subjected to flash silica gel column chromatography to obtain the multi-substituted benzoate compound 3a.
Embodiment 2
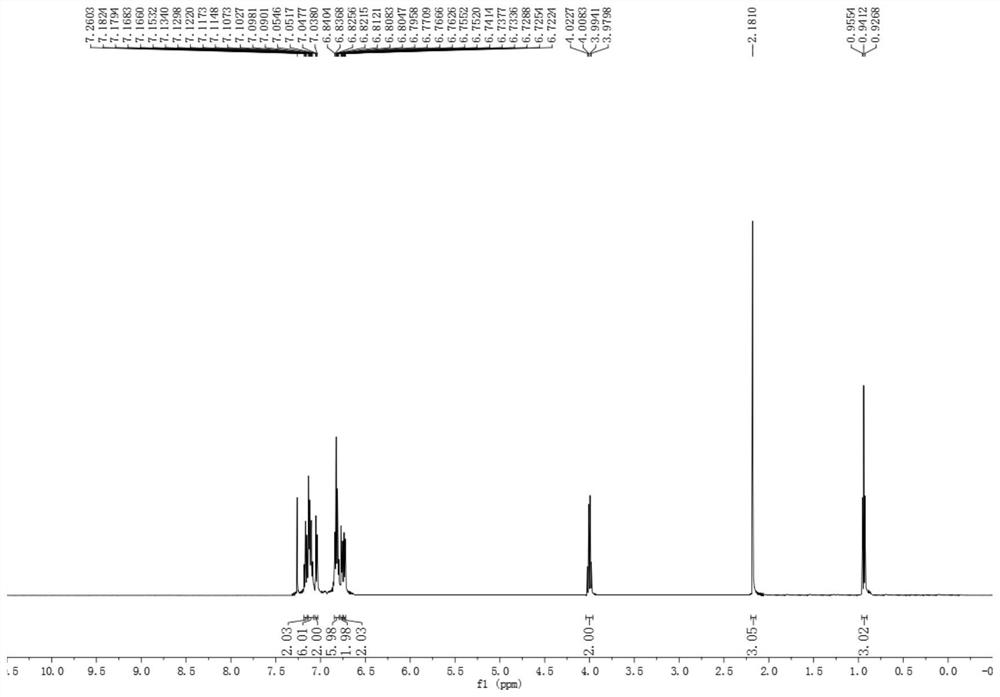
[0031] Add 3mL of toluene to a 10mL round bottom flask, then add 3mmol methyl acetoacetate and 1mmol toluene and mix well to obtain a mixed solution. 7% cuprous iodide of the total mass of the mixed solution was added as a catalyst to form a reaction system. The reaction system was heated to 100 degrees Celsius, and the reaction was stirred for 20 h under the condition that the temperature of the reaction system was kept at 100 degrees Celsius. After the reaction, the reaction product obtained was extracted three times with ethyl acetate and water 30mL (volume ratio 2:1), and a certain amount of anhydrous magnesium sulfate was added to the obtained upper organic phase to stand overnight for drying, and the solvent toluene was evaporated under reduced pressure. The crude product was obtained, and the crude product was subjected to flash silica gel column chromatography to obtain the multi-substituted benzoate compound 3b.
Embodiment 3
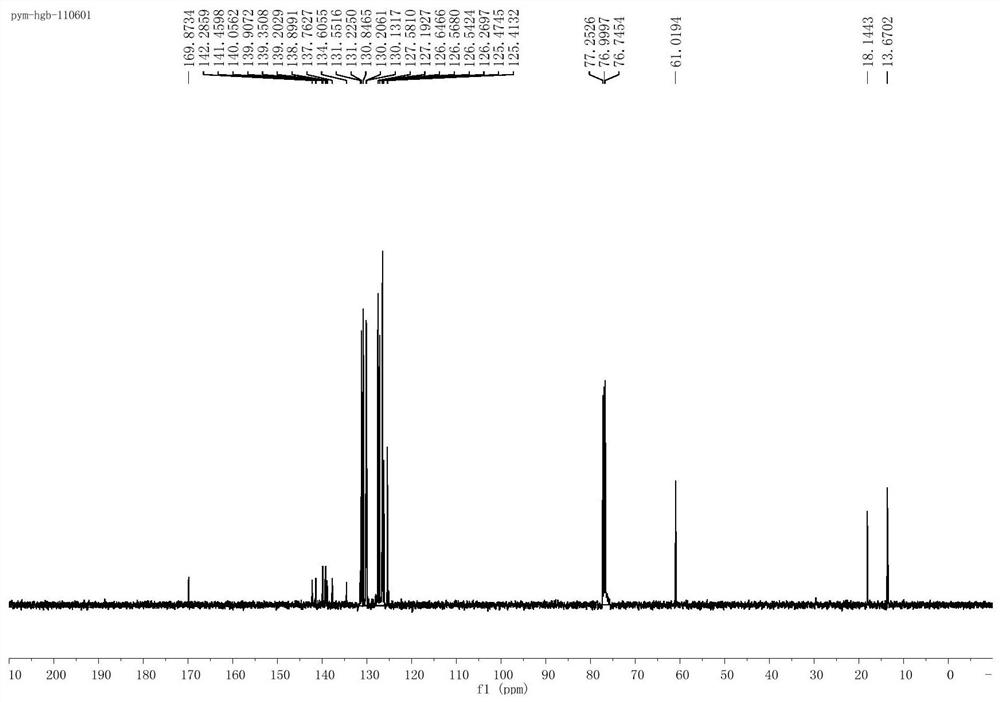
[0033] Add 3mL of toluene to a 10mL round bottom flask, then add 5mmol isopropyl acetoacetate and 1mmol toluene and mix well to obtain a mixed solution. 10% cuprous bromide of the total mass of the mixed solution is added as a catalyst to form a reaction system. The reaction system was heated to 110 degrees Celsius, and the reaction was stirred for 24 hours under the condition that the temperature of the reaction system was kept at 110 degrees Celsius. After the reaction, the reaction product obtained was extracted three times with ethyl acetate and water 30mL (volume ratio 2:1), and a certain amount of anhydrous magnesium sulfate was added to the obtained upper organic phase to stand overnight for drying, and the solvent toluene was evaporated under reduced pressure. The crude product was obtained, and the crude product was subjected to flash silica gel column chromatography to obtain the multi-substituted benzoate compound 3c.
[0034] The structural formulas of the above-m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com