Preparation method of fluorinated nano-graphite as cathode material for lithium fluoride battery
A lithium carbon fluoride battery and positive electrode material technology, which is applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of material performance impact, material embrittlement and cracking, and long graphene preparation process, so as to achieve guaranteed effect and improve fluorine The effect of content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Preparation of carbon precursor: Grind flake graphite and steel needles with magnetic stirring for 6 h under the protection of inert gas, cool down to room temperature after grinding, separate nano-graphite and steel needles by sieving, and perform high-temperature heat treatment on nano-graphite. During high temperature heat treatment, the heating rate from room temperature to treatment temperature is 5 ℃∙min -1 , the high temperature heat treatment temperature is 800 ℃, the treatment time is 2 h, and the carbon precursor is obtained;
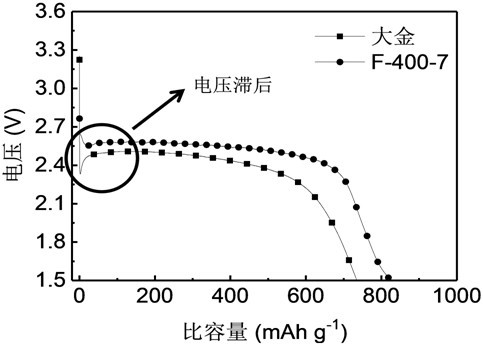
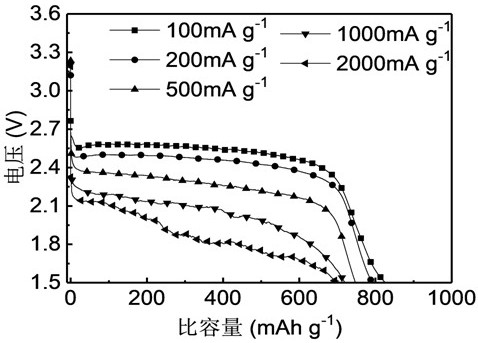
[0024] 2) Fluorination: Spread the nano-graphite flat into the high-temperature reactor, blow nitrogen and start heating, the heating rate is 5 ℃ min -1 After one hour, the reaction was replaced by fluorine gas, the reaction temperature was 400 °C, and the reaction time was 7 h. After the reaction, the fluorine gas was replaced by nitrogen gas for 12 h and cooled to room temperature to obtain the fluorinated nano-graphite material F-...
Embodiment 2
[0026] 1) Preparation of carbon precursor: Grind flake graphite and steel needles with magnetic stirring for 5.7 h under the protection of inert gas, cool down to room temperature after grinding, separate nano-graphite and steel needles by sieving, and perform high-temperature heat treatment on nano-graphite. During high temperature heat treatment, the heating rate from room temperature to treatment temperature is 4.8 ℃∙min -1 , the high temperature heat treatment temperature is 780 ℃, the treatment time is 2.1 h, and the carbon precursor is obtained;
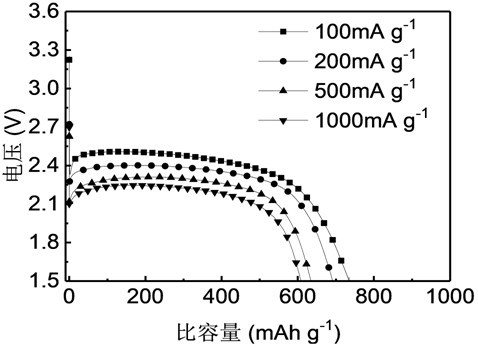
[0027] 2) Fluorination: spread the nano-graphite into the high-temperature reactor, pass nitrogen gas and start heating, the heating rate is 5 ℃ / min, and change to fluorine gas for reaction after one hour, the reaction temperature is 450 ℃, and the reaction time is 6.2 h, after the reaction, replace the fluorine gas with nitrogen for 12 h and cool down to room temperature to obtain the fluorinated nano-graphite material F-450-7...
Embodiment 3
[0029] 1) Preparation of carbon precursor: Grind flake graphite and steel needles with magnetic stirring under the protection of inert gas for 4.5 h to 7.5 h, cool down to room temperature after grinding, separate nano-graphite and steel needles by sieving, and then grind nano-graphite High temperature heat treatment, the heating rate from room temperature to treatment temperature during high temperature heat treatment is 5.2 ℃∙min -1 , the treatment temperature of high temperature heat treatment is 820 ℃, and the treatment time is 1.9 h to obtain the carbon precursor;
[0030] 2) Fluorination: Spread the nano-graphite flat into the high-temperature reactor, pass nitrogen gas and start heating, the heating rate is 5°C / min, and change to fluorine gas for reaction after one hour, the reaction temperature is 425°C, and the reaction time is 6.3 h, after the reaction, replace the fluorine gas with nitrogen for 12 h and cool down to room temperature to obtain the fluorinated nano-gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com