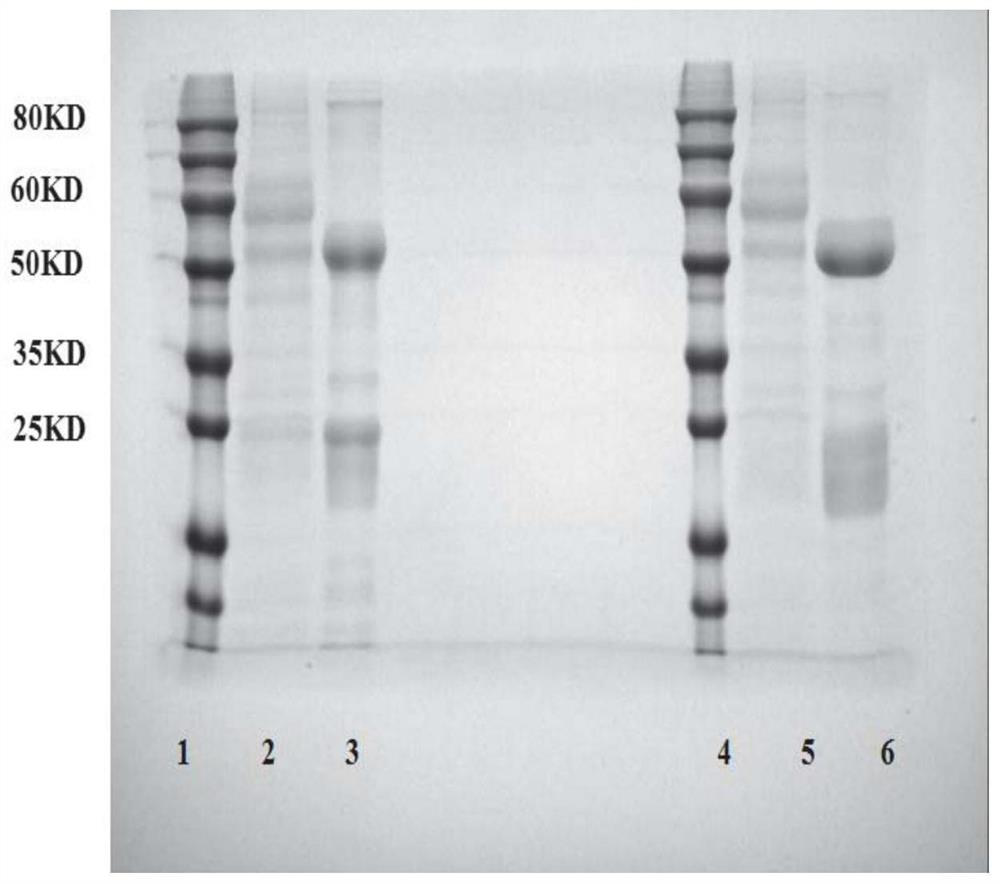
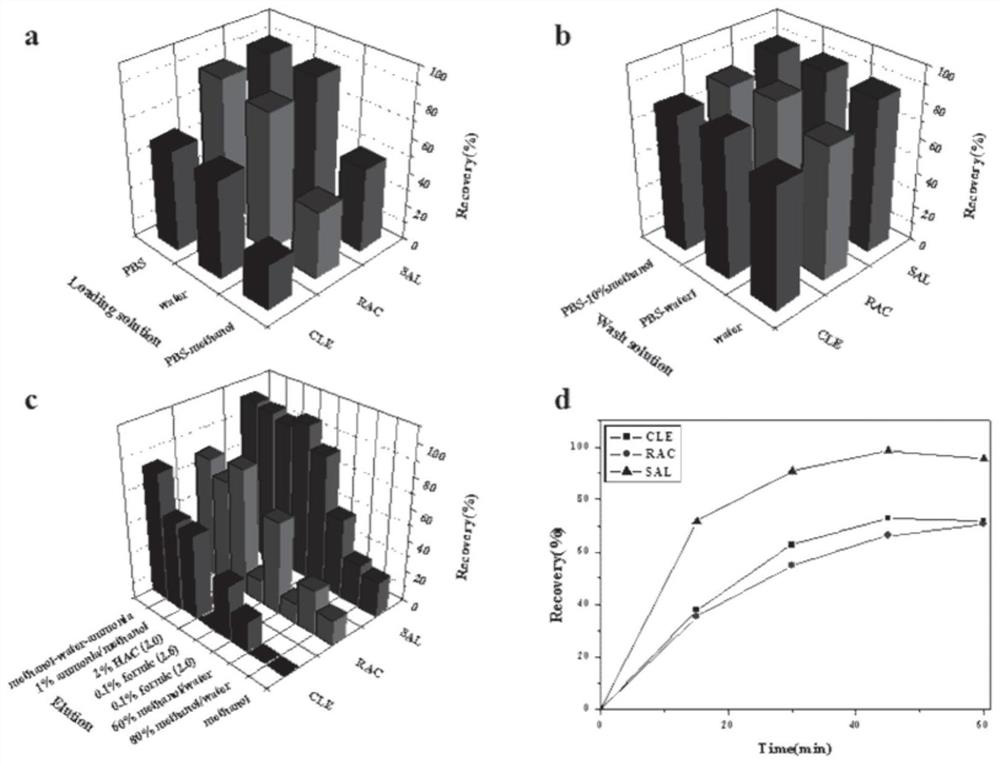
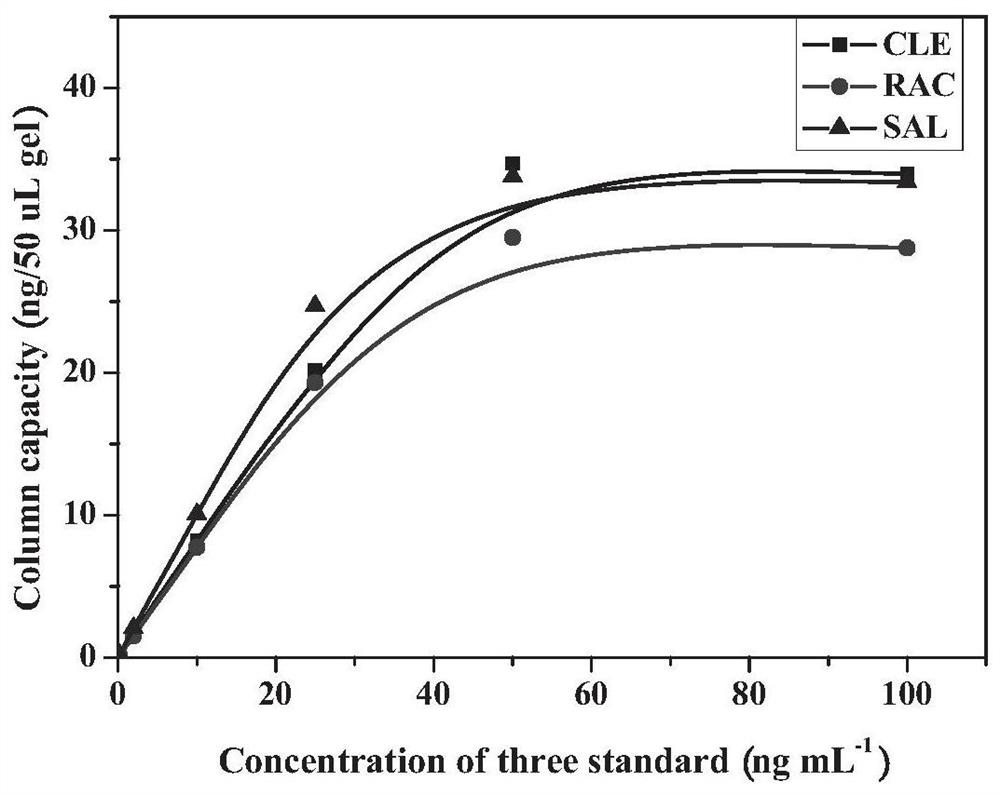
An anti-beta-receptor agonist cluster-specific monoclonal antibody hybridoma cell line and its secreted monoclonal antibody and application
A hybridoma cell line, receptor agonist technology, applied in the biological field, can solve the problems of limited application, difficult, time-consuming and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Acquisition of ascites samples containing monoclonal antibodies according to the present invention
[0071] 1. Recovery and proliferation of hybridoma cell line 1C3A and patent CN104311438A hybridoma cells
[0072] Take the cryopreservation tube of the hybridoma cell line from the liquid nitrogen tank, thaw it quickly in a water bath at 37°C, centrifuge at 600r / min for 5min, discard the supernatant, add 15% FBS / RPMI-1640 culture medium to suspend the cells, and add the above After the culture solution reaches 5ml, plant it in a 50ml cell culture bottle and culture it in a carbon dioxide incubator. After the cells grow to 30% density, change the medium halfway. - Passage once every 3 days according to 1:3-4.
[0073] 2. In vivo induction of monoclonal antibody protein and ascites acquisition
[0074] 7-10 days before planting hybridoma cells in vivo, 12 male BALB / c mice aged 8-10 weeks were injected intraperitoneally with 0.5ml / mouse of pristane, and careful...
Embodiment 2
[0077] Example 2: Purification of monoclonal antibody protein of the present invention and ractopamine monoclonal antibody of patent CN104311438A hybridoma cells
[0078] Aspirate 2mL protein G resin suspension, transfer it to a disposable PE column with a column capacity of 12mL, then add 20mL binding buffer (3.3768g Na 2 HPO 4 12H 2 O, 0.0888g NaH 2 PO 4 2H 2 O, 8.5g NaCl, 1LH 2 O preparation), the flow rate is 1mL min -1 , to remove impurities in the suspension and to precipitate the suspension. Then slowly add 4mL of monoclonal antibody ascites to make it slowly flow through the gel layer. Protein G is the cell wall protein of group G streptococci, which can capture the Fc region of IgG antibodies, while other subtypes cannot be captured, so as to achieve the purpose of purification . After the ascites has completely flowed through the gel layer, add 100 mL of binding buffer to wash to remove non-specific adsorption, and then add 15 mL of glycine buffer (0.1 mol L ...
Embodiment 3
[0080] Example 3: Analysis of Immune Crossover
[0081] The present invention selects clenbuterol hydrochloride, terbutaline, bromobuterol, sibuterol, and these four beta-adrenoceptor agonists to detect the cross-reaction rate. Albuterol concentration range 0-30ng mL -1 , the concentration range of cross-products is 0.1-10000ng mL -1 . Make standard curves for albuterol and other 4 cross-products, and calculate their respective ICs 50 , so as to obtain their respective CR values, and the results are shown in Table 1.
[0082] Table 1
[0083]
[0084] As can be seen from Table 1, when the CR value of albuterol was determined to be 100%, it was found that the cross-reaction rates of clenbuterol hydrochloride, terbutaline, bromobuterol and sibuterol were very large, and the CR values were respectively 66.8%, 57.3%, 42.1%, 121.5%. These four substances are very similar to salbutamol in structure, and all have a tert-butyl group connected to a nitrogen atom, indicating ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com