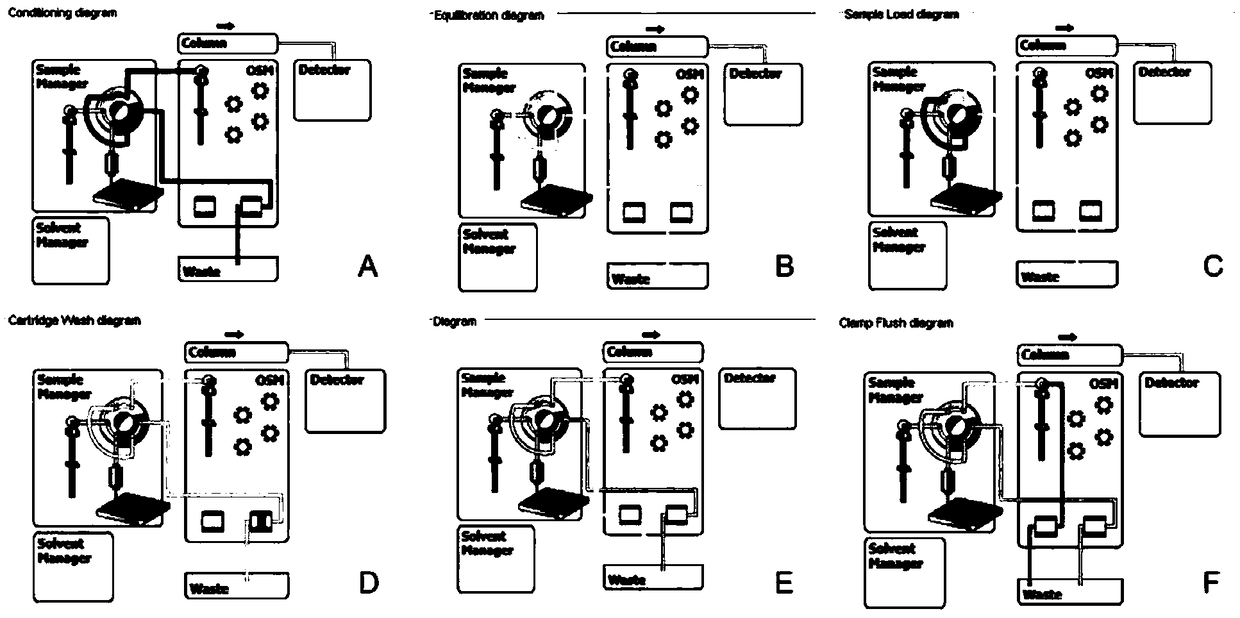
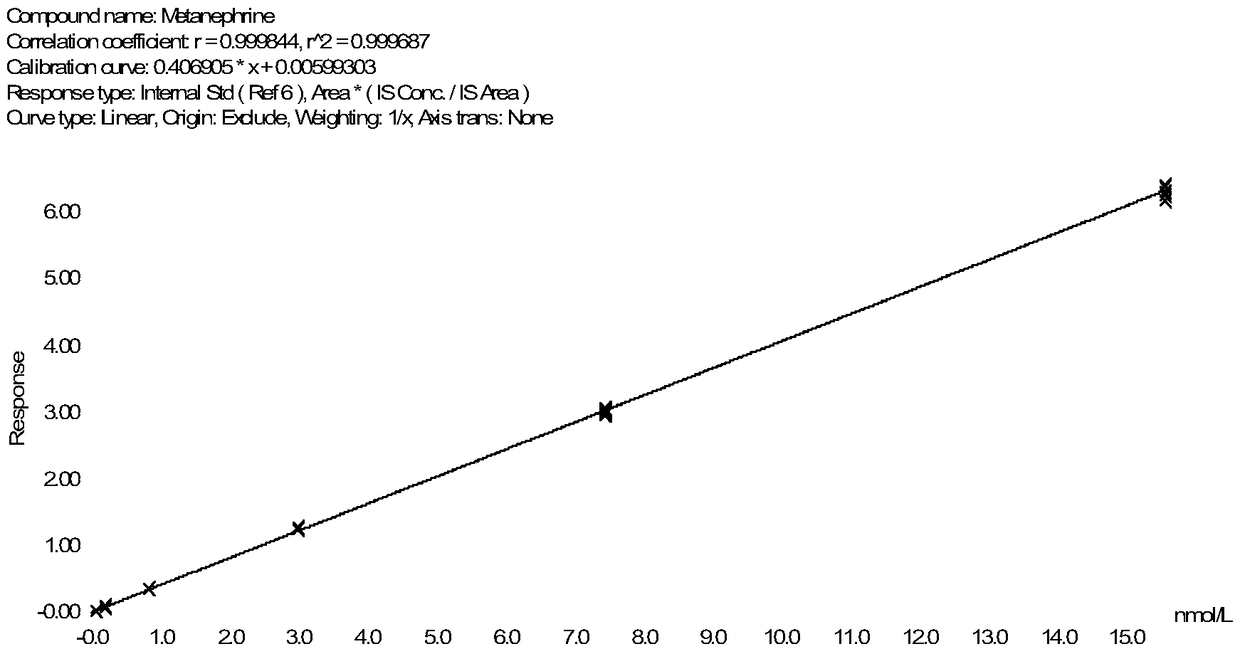
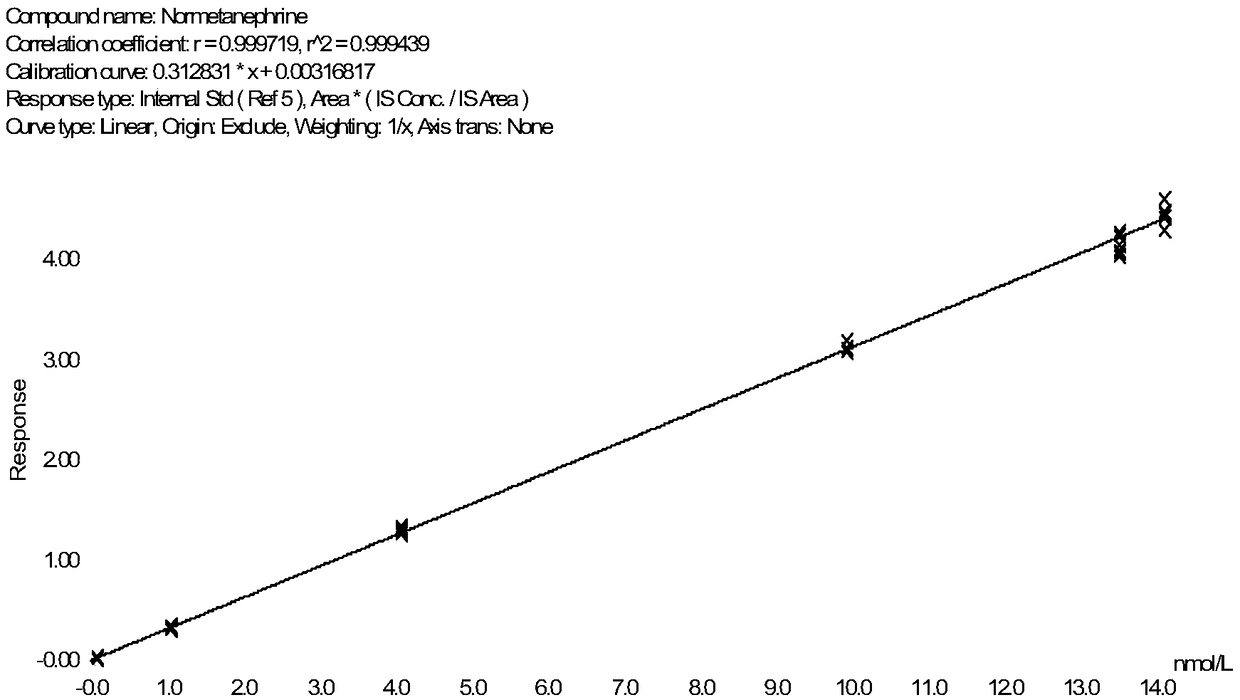
Method for detecting metanephrines through liquid chromatography-tandem mass spectrometry
A methoxyepinephrine and tandem mass spectrometry technology, applied in the field of medical testing, can solve the problems of quantitative analysis of 3-methoxytyramine, high cost of ultrafiltration tubes, and long training period, so as to reduce manual solid phase extraction operations, Removal of interfering substances and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] An embodiment of the method for detecting methoxyadrenaline substances by liquid chromatography-tandem mass spectrometry of the present invention comprises the following steps:
[0062] (1) Sample pretreatment: Take 200 μL plasma sample in a 1.5 mL centrifuge tube, add 800 μL mixed internal standard solution, shake and centrifuge, transfer 900 μL supernatant to 1 mL 96-well adapter plate, blow dry with nitrogen at 40°C, add 300 μL 5mmol / L phosphate buffer solution to obtain a complex solution for use;
[0063] The mixed internal standard solution is an acetonitrile solution containing an internal standard of methoxyepinephrine, an internal standard of methoxynorepinephrine and an internal standard of 3-methoxytyramine; The substance is d3-methoxyepinephrine, the methoxynorepinephrine internal standard substance is d3-methoxynoradrenaline, and the 3-methoxytyramine internal standard substance is d4-3-formazan Oxytyramine; the concentrations of the d3-methoxyepinephrine...
Embodiment 2
[0084] An embodiment of the method for detecting methoxyadrenaline substances by liquid chromatography-tandem mass spectrometry of the present invention comprises the following steps:
[0085] (1) Sample pretreatment: Take 200 μL plasma sample in a 1.5 mL centrifuge tube, add 600 μL mixed internal standard solution, shake and centrifuge, transfer 500 μL supernatant to a 1 mL 96-well plate, blow dry with nitrogen at 70°C, add 200 μL 15 mmol / L Phosphate buffer saline, to obtain complex solution, stand-by;
[0086] The mixed internal standard solution is an acetonitrile solution containing an internal standard of methoxyepinephrine, an internal standard of methoxynorepinephrine and an internal standard of 3-methoxytyramine; The substance is d3-methoxyepinephrine, the methoxynorepinephrine internal standard substance is d3-methoxynoradrenaline, and the 3-methoxytyramine internal standard substance is d4-3-formazan Oxytyramine; the concentrations of the d3-methoxyepinephrine, d3-...
Embodiment 3
[0100] An embodiment of the method for detecting methoxyadrenaline substances by liquid chromatography-tandem mass spectrometry of the present invention comprises the following steps:
[0101] (1) Sample pretreatment: Take 200 μL plasma sample in a 1.5 mL centrifuge tube, add 700 μL mixed internal standard solution, shake and centrifuge, transfer 600 μL supernatant to a 1 mL 96-well plate, blow dry with nitrogen at 60°C, add 400 μL 20 mmol / L Phosphate buffer saline, to obtain complex solution, stand-by;
[0102] The mixed internal standard solution is an acetonitrile solution containing an internal standard of methoxyepinephrine, an internal standard of methoxynorepinephrine and an internal standard of 3-methoxytyramine; The substance is d3-methoxyepinephrine, the methoxynorepinephrine internal standard substance is d3-methoxynoradrenaline, and the 3-methoxytyramine internal standard substance is d4-3-formazan Oxytyramine; the concentrations of the d3-methoxyepinephrine, d3-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com