Clindamycin phosphate freeze-drying powder for injection, and preparation method thereof
A technology of clindamycin phosphate and freeze-dried powder, which is applied in the field of clindamycin phosphate freeze-dried powder and its preparation, and can solve the problems such as change in appearance of clindamycin phosphate freeze-dried powder, increase in substance content and the like , to achieve the effect of good resolubility, little muscle stimulation, and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
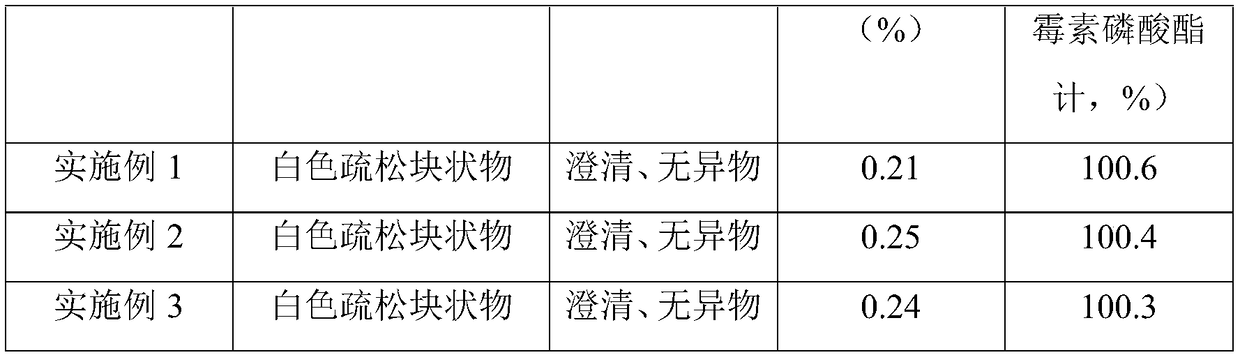
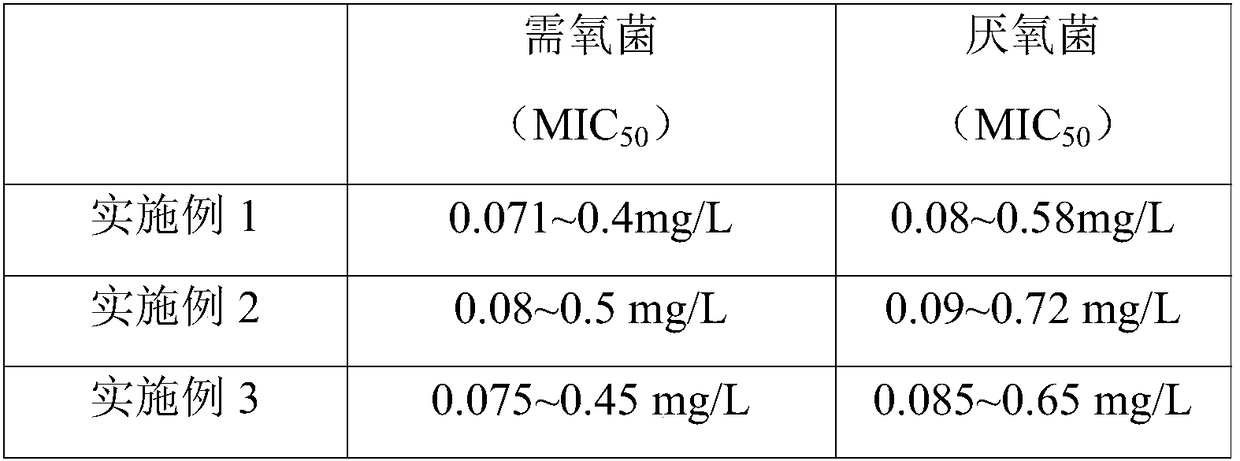
[0034] A clindamycin phosphate freeze-dried powder for injection, wherein the main drug is clindamycin phosphate, the auxiliary solvent is sodium hydroxide, the auxiliary materials are sodium chloride and mannitol, and the solvent is water for injection. The quality of the clindamycin phosphate, sodium chloride, mannitol and sodium hydroxide is 300g, 30g, 12g and 54g respectively, which is supplemented to 2200g with water for injection. Use the above-mentioned materials to make 1000 finished products with a specification of 0.3g.
[0035] Its preparation process includes:
[0036] S1, material preparation: weigh clindamycin phosphate, sodium chloride, mannitol, sodium hydroxide according to the formula;
[0037] S2, preparation of medicinal solution: add 1100g water for injection to the liquid preparation tank, add 54g sodium hydroxide to fully dissolve, cool to room temperature; then add 300 grams of lindamycin phosphate, 30 g sodium chloride and 12 g Mannitol, stir to make...
Embodiment 2
[0048] The difference between this embodiment and embodiment 1 is:
[0049] During preparation,
[0050] S2, preparation of medicinal solution: add 1100g water for injection to the liquid preparation tank, add 54g sodium hydroxide to fully dissolve, cool to room temperature; then add 300 grams of lindamycin phosphate, 30 g sodium chloride and 12 g Mannitol, stir to make it dissolve completely; take a sample to test the pH value of the medicinal solution, adjust the pH value of the system to 6.0 with 1mol / L sodium hydroxide solution; then make up to 2200g with water for injection;
[0051] S5. Freeze-drying: put the subpackaged vials into a freeze-dryer, and freeze-dry to obtain a freeze-dried powder; the specific drying steps include:
[0052] S51. Pre-freezing: place the subpackaged clindamycin phosphate liquid on the partition layer in the lyophilizer, adjust the temperature of the partition, and reduce the clindamycin phosphate liquid to -20°C. Keep warm for 30 minutes; t...
Embodiment 3
[0058] The difference between this embodiment and embodiment 1 is:
[0059] A kind of freeze-dried powder of clindamycin phosphate for injection, the quality of described clindamycin phosphate, sodium chloride, mannitol, sodium hydroxide is respectively 300g, 33g, 10.5g, 60g, supplemented with water for injection to 2200g.
[0060] The difference in the preparation process is that,
[0061] S2, preparation of medicinal liquid: add 1100g water for injection in the liquid preparation tank, add 60g sodium hydroxide to fully dissolve, cool to room temperature; then add 300 grams of lindamycin phosphate, 33g sodium chloride and 10.5 g mannitol, stir to make it dissolve completely; take a sample to test the pH value of the medicinal solution, adjust the pH value of the system to 5.8 with 1mol / L sodium hydroxide solution; then make up to 2200g with water for injection;
[0062] S3. Filtration: add 4.4g of medicinal activated carbon to the liquid mixing tank, stir and absorb for 35m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com