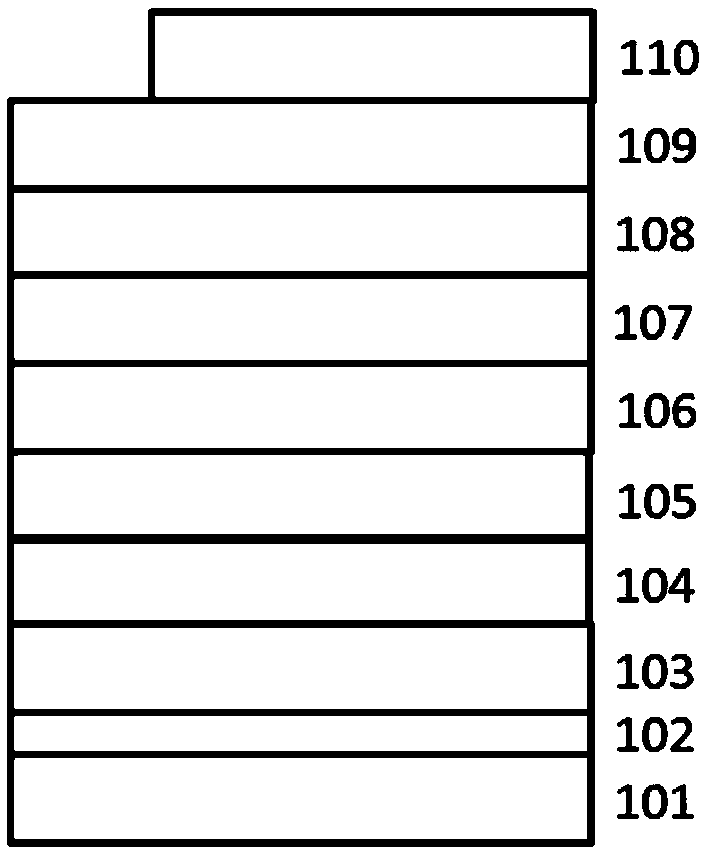
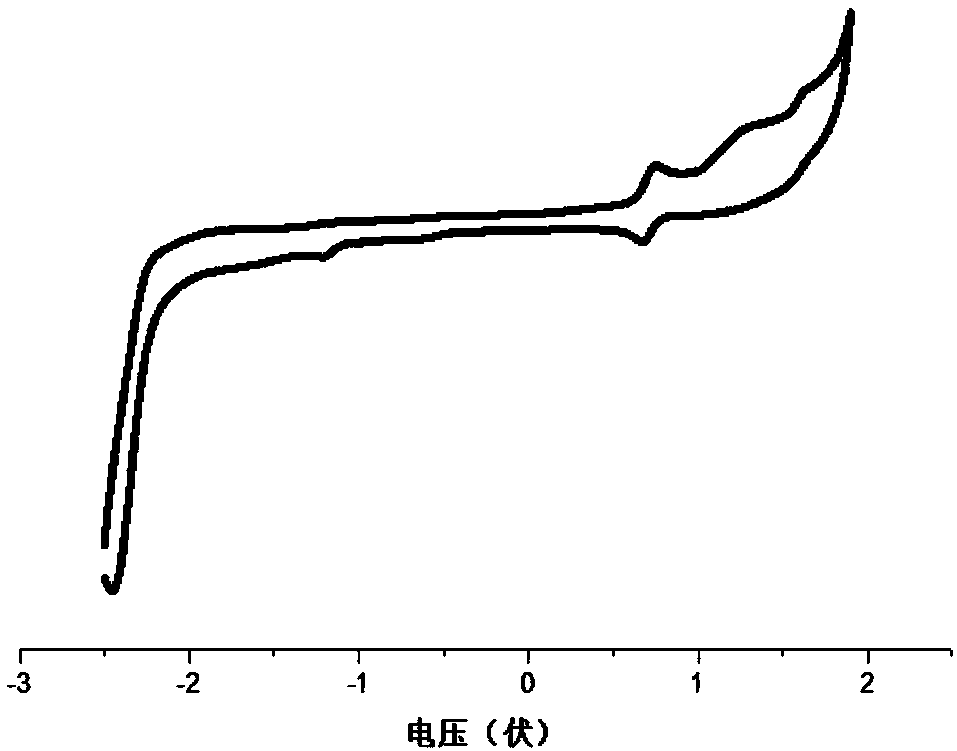
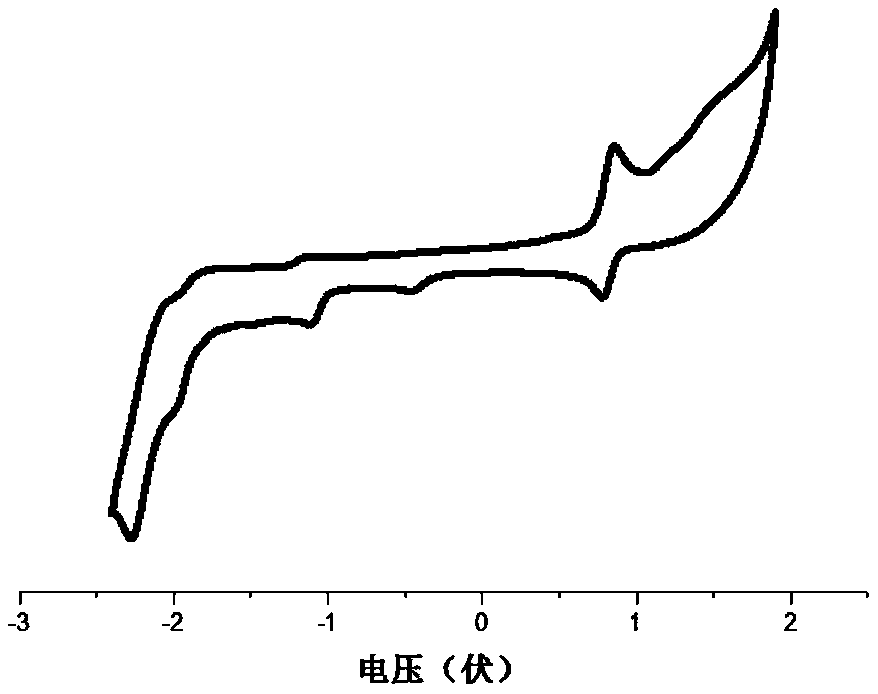
Polyaza-spirobifluorene compound and organic optoelectronic device using the same
A compound, azaspiro technology, applied in the field of organic optoelectronics, to achieve the effect of improving efficiency, simplifying structure, and improving luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] Preparation routes of key intermediate materials
[0050]
[0051] Preparation of M1:
[0052]
[0053] Add 3-bromo-2-(2-chlorophenyl)pyridine (13g) and 130mL anhydrous tetrahydrofuran into a dry three-necked flask, replace with nitrogen and cool down to -78°C with liquid nitrogen, then add 20mL of 2.5M n-butyllithium, keep at -78°C for half an hour, TCL detects that the reaction is complete, then dissolve 10g of chloroazafluorenone in 100ml of tetrahydrofuran, add it to the above three-necked flask, and keep at -78°C After reacting for half an hour, the reaction was detected by TCL, and the temperature was raised to room temperature, then quenched with 10% dilute hydrochloric acid solution, extracted with dichloromethane and then spin-dried to obtain the crude product of fluorenol. The resulting crude product of fluorenol was then directly added to 200ml of CH 3 COOH, and then added 10ml of concentrated sulfuric acid, and reacted overnight under reflux conditio...
Embodiment 1
[0056] Embodiment 1: the synthesis of compound 1-44-1
[0057]
[0058] M1 (1.8g) and 3-phenylphenylboronic acid (5g) were completely dissolved in 120ml tetrahydrofuran in a 250ml round-bottomed flask under a nitrogen atmosphere, then 60ml of 2M aqueous sodium carbonate was added, and then tetrakis-(triphenyl Phosphine)palladium (0.3g), and the mixture was heated and stirred for 12 hours. After cooling to room temperature, the aqueous layer was removed. Add 100 ml of dichloromethane, and wash twice with 30 ml of saturated brine. The dichloromethane layer was dried over anhydrous magnesium sulfate, and concentrated in vacuo. Then dichloromethane:ethyl acetate (20:1~2:1) was used as eluent to purify and separate on a silica gel column to obtain 1-44-1 (yield 82%); MS (ESI): 623.2 ( M+H)
Embodiment 2
[0059] Embodiment 2: the synthesis of compound 1-44-49
[0060] MS (ESI): 933.3 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com