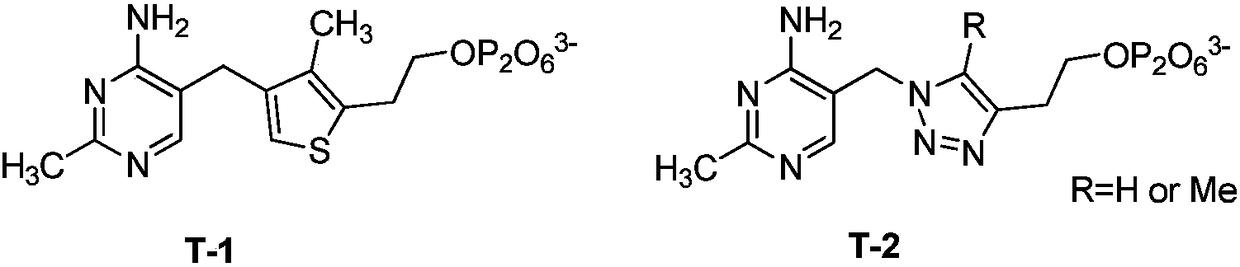
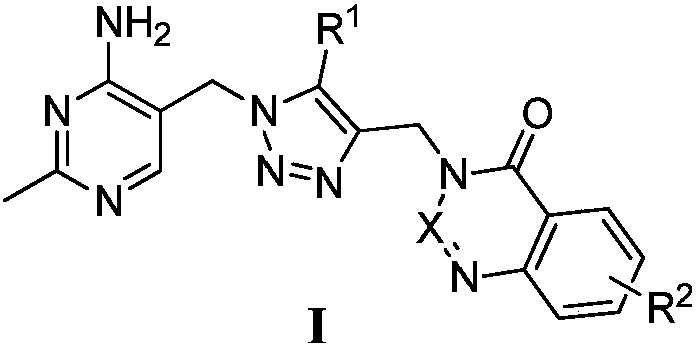
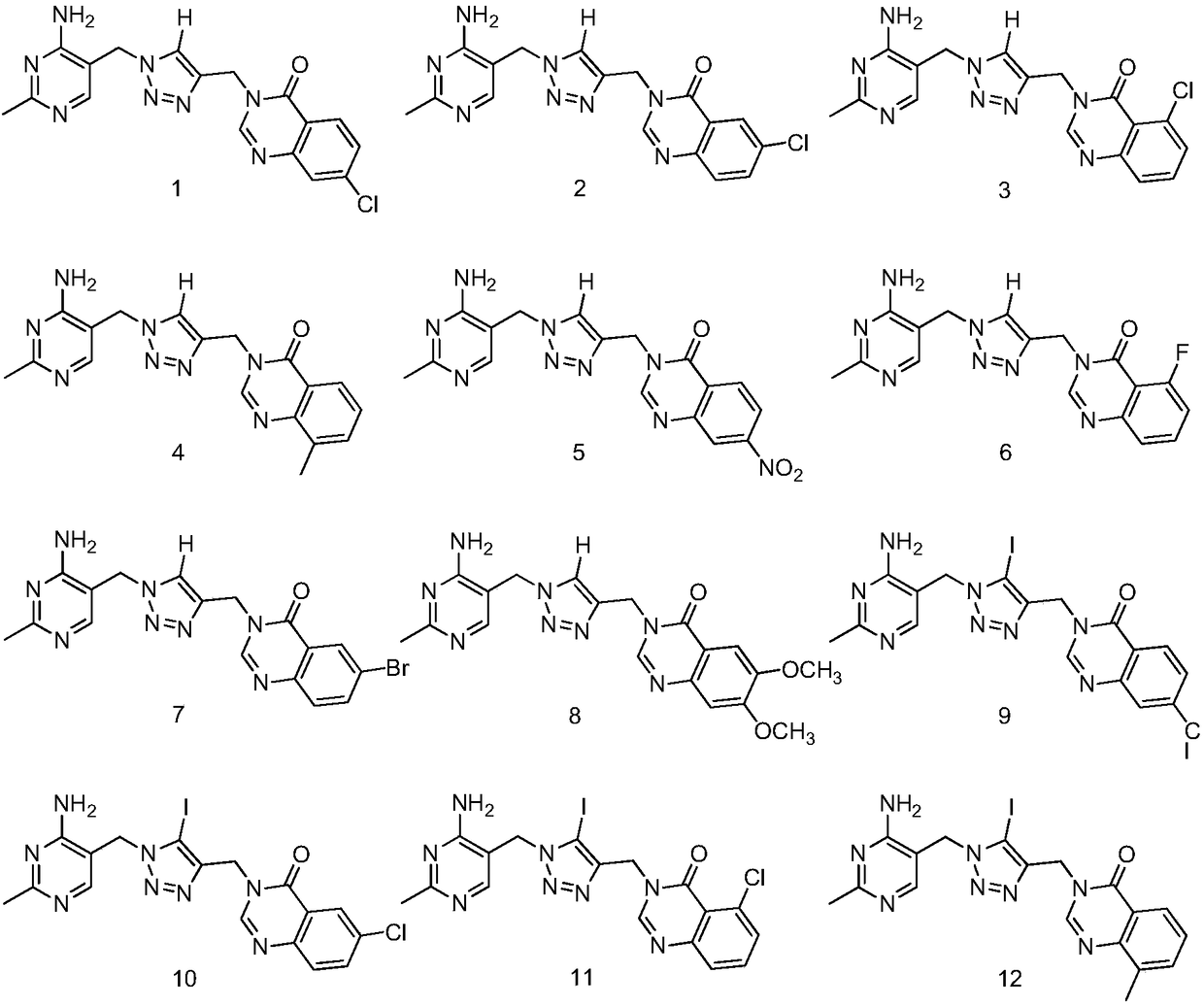
Branched-chain alpha-keto-acid dehydrogenase system inhibitor and preparation method and application thereof
A catalyst and solvate technology, applied in the field of chemistry, can solve problems such as complex structure of compounds, no application value, and difficult synthesis, and achieve the effect of simple and easy control of the reaction operation process, favorable for mass preparation, and high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] Preparation of compound 1:
[0052] Dissolve 1mmol of 2-methyl 4-amino-5-methylpyrimidine azido and 1mmol of 4-chloroquinazolone amine propyne in 6ml of tert-butanol and water (volume ratio of tert-butanol to water=2:1) Add 0.01 mmol of copper sulfate pentahydrate and 0.1 mmol of sodium ascorbate to the solvent, react at room temperature for 12-24 hours, add 50 ml of water to the reaction system, stir to precipitate solids, suction filter, and dry to obtain yellow solids. Yield 86%, mp 222-224°C;
[0053] 1 H NMR (400MHz, DMSO-d 6 )δ(ppm): 2.29(s,3H),5.22(s,2H),5.38(s,2H),6.87(s,2H), 7.56(d,J=8.7Hz,1H),7.73(s, 1H), 8.10(s, 2H), 8.56(s, 1H);
[0054] HRMS (ESI): calcd.for C 17 h 15 ClN 8 O[M+H] + 383.11301, found: 383.11267.
[0055] Compound 2-14 was prepared in a similar manner to compound 1, and its structural identification data are as follows.
Embodiment 2
[0057]
[0058] The obtained pure product is a white solid with a yield of 89%, m.p.209-211°C;
[0059] 1 H NMR (400MHz, DMSO-d 6 )δ(ppm): 2.29(s,3H),5.23(s,2H),5.38(s,2H),6.87(s,2H), 7.69(s,1H),7.83(s,1H),8.07( d,J=23.3Hz,2H),8.54(s,1H);
[0060] HRMS (ESI): calcd.for C 17 h 15 ClN 8 O[M+H] + 383.11301, found: 383.11274.
Embodiment 3
[0062]
[0063] The obtained pure product is a white solid with a yield of 82%, m.p.133-135°C;
[0064] 1 H NMR (400MHz, DMSO-d 6 )δ(ppm): 2.29(s,3H),5.18(s,2H),5.39(s,2H),6.88(s,2H), 7.57(d,J=22.6Hz,2H),7.72(s, 1H), 8.12(s,1H), 8.54(s,1H);
[0065] HRMS (ESI): calcd.for C 17 h 15 ClN 8 O[M+H] + 383.11301, found: 383.11292.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com