Preparation and application of a porcine Japanese encephalitis vaccine composition
A vaccine composition, Japanese encephalitis virus technology, applied in the directions of vaccines, veterinary vaccines, medical preparations containing active ingredients, etc. Effects of swine Japanese encephalitis, increasing antibody levels, and enhancing immune response effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 vaccine composition of the present invention
[0032] (1) Preparation of attenuated Japanese encephalitis Japanese encephalitis vaccine:
[0033] The JEV SCYA201201 attenuated strain was inoculated into BHK-21 cells (obtained by conventional cell culture), and the virus liquid was harvested. The specific method is as follows:
[0034] ①Select the BHK-21 cells that grow into a dense monolayer, wash twice with PBS, inoculate JEV SCYA201201 attenuated strain, the inoculum volume is 0.4mL, and incubate at 37°C.
[0035] ②Shake every 15 minutes during the incubation period, pour out the virus solution after 1 hour, add 6mL of maintenance solution (the maintenance solution is DMEM culture solution), and place at 37°C, 5% CO 2 Cultivate in a constant temperature incubator, and observe whether the cells have lesions every day.
[0036] ③ When 80% to 90% of the cells have lesions, freeze and thaw the cell culture repeatedly 3 times, pipette repe...
Embodiment 2
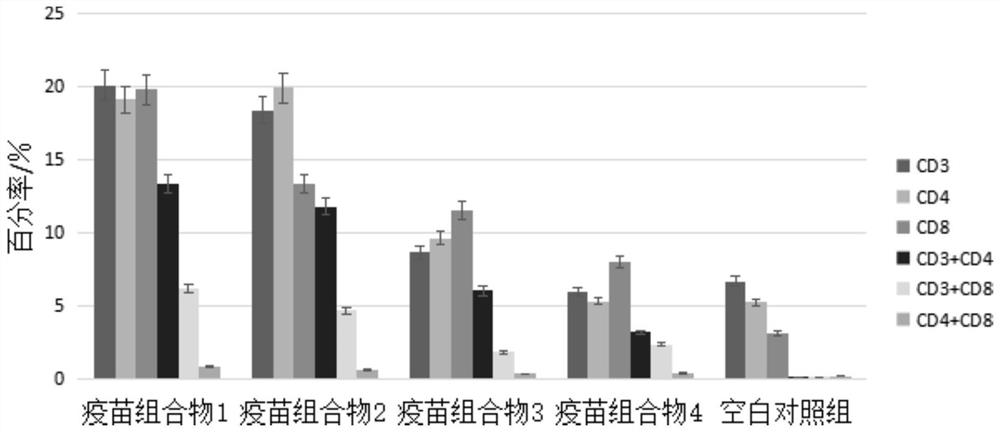
[0040] Example 2 The challenge protection of the vaccine composition of the present invention to mice
[0041] 1. Test material
[0042] Vaccine composition 1: take 10 ml of the vaccine composition prepared in Example 1, and the adjuvant concentration is 14.4 mg / mL (W / V).
[0043] Vaccine composition 2: Take 5ml of the double dilution of the stock solution prepared in Example 1, add 5ml of SCYA201201 attenuated strain cell culture solution, and the final concentration of adjuvant is 7.2mg / mL (W / V).
[0044] Vaccine composition 3: Take 5ml of the four-fold dilution of the stock solution prepared in Example 1, add 5ml of SCYA201201 attenuated strain cell culture solution, and the final concentration of adjuvant is 3.6mg / mL (W / V).
[0045] Vaccine composition 4: Take 5 ml of phosphate buffered saline (PBS, pH=7.2), add 5 ml of SCYA201201 attenuated strain cell culture medium, and the final concentration of adjuvant is 0 mg / mL (W / V).
[0046] 2. Test method
[0047] A total of ...
Embodiment 3
[0056] Embodiment 3 The antibody level detection of vaccine composition of the present invention
[0057] 1. Test material
[0058] Vaccine composition 1: take 10 ml of the vaccine composition prepared in Example 1, and the adjuvant concentration is 14.4 mg / mL (W / V).
[0059] Vaccine composition 2: Take 5ml of the double dilution of the stock solution prepared in Example 1, add 5ml of SCYA201201 attenuated strain cell culture solution, and the final concentration of adjuvant is 7.2mg / mL (W / V).
[0060] Vaccine composition 3: Take 5ml of the four-fold dilution of the stock solution prepared in Example 1, add 5ml of SCYA201201 attenuated strain cell culture solution, and the final concentration of adjuvant is 3.6mg / mL (W / V).
[0061] Vaccine composition 4: Take 5 ml of phosphate buffered saline (PBS, pH=7.2), add 5 ml of SCYA201201 attenuated strain cell culture medium, and the final concentration of adjuvant is 0 mg / mL (W / V).
[0062] 2. Test method
[0063] A total of 50 3-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com