Preparation method of phenoxyacetic ester
A technology of phenoxyacetate and acetate, applied in the field of preparation of phenoxyacetate, can solve problems such as affecting product quality and being difficult to separate, and achieves the effects of low production cost, short production cycle and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
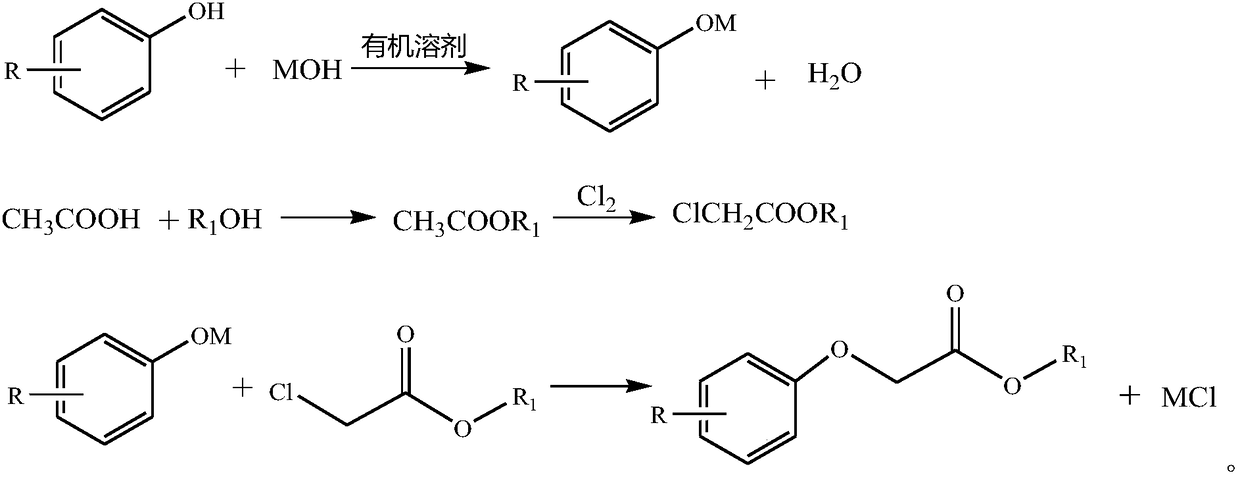
[0053] Add acetic acid and methanol into the reactive distillation column to synthesize methyl acetate.
[0054] Add the synthesized methyl acetate into the reaction flask and feed chlorine gas to synthesize methyl chloroacetate.
[0055] 47.6g (0.5mol) of phenol, 120g of xylene, 30g of butanol, and 62.8g (0.5mol) of sodium hydroxide aqueous solution with a mass fraction of 32% were mixed and stirred, heated to about 140°C, and reacted with water for 1 hour. The neutralized solution was slightly cooled to 120°C, 53.8 g (0.495 mol) of methyl chloroacetate was added dropwise, and the addition was completed in 4 hours, then the temperature was raised to 146°C, and the reaction was kept for 2 hours. Cool the reaction solution to 100°C, add 150g of water, adjust the pH to 7, let stand to separate layers, add 20g of xylene to the water layer for extraction, and separate the organic layer by vacuum distillation to obtain 81.75g of the product with a purity of 99.1% and a yield of 97....
Embodiment 2
[0057] Add acetic acid and methanol into the reactive distillation column to synthesize methyl acetate.
[0058] Add the synthesized methyl acetate into the reaction flask and feed chlorine gas to synthesize methyl chloroacetate.
[0059] Mix and stir 109.5 g (1 mol) of o-cresol, 350 g toluene, 100 g butanol, and 125.2 g (1 mol) of sodium hydroxide aqueous solution with a mass fraction of 32%, raise the temperature to about 114°C, and react with water for 1 hour. The neutralized solution was slightly cooled to 120°C, 107.7 g (0.98 mol) of methyl chloroacetate was added dropwise, and the addition was completed in 4 hours, then the temperature was raised to 116°C, and the reaction was kept for 2 hours. Cool the reaction solution to 100°C, add 150g of water, adjust the pH to 7, let stand to separate layers, add 40g of toluene to the water layer for extraction, and separate the organic layer by vacuum distillation to obtain 163.35g of the product with a purity of 99.13% and a yiel...
Embodiment 3
[0061] Add acetic acid and methanol into the reactive distillation column to synthesize methyl acetate.
[0062] Add the synthesized methyl acetate into the reaction flask and feed chlorine gas to synthesize methyl chloroacetate.
[0063] Mix and stir 95.5 g (1 mol) of phenol, 350 g toluene, 100 g butanol, and 125.2 g (1 mol) of sodium hydroxide aqueous solution with a mass fraction of 32%, raise the temperature to about 114°C, and react with water for 1 hour. The neutralized solution was slightly cooled to 120°C, 109.8 g (0.99 mol) of methyl chloroacetate was added dropwise, and the addition was completed in 4 hours, then the temperature was raised to 116°C, and the reaction was kept for 2 hours. Cool the reaction solution to 100°C, add 150g of water, adjust the pH to 7, let stand to separate layers, add 40g of toluene to the water layer for extraction, and separate the organic layer by vacuum distillation to obtain 163.35g of the product with a purity of 99.2% and a yield of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com