Method for recycling stainless steel pickling wastewater
A technology for pickling wastewater and stainless steel, applied in chemical instruments and methods, metallurgical wastewater treatment, water/sewage treatment, etc., can solve the problems of difficult heavy metal sludge treatment, low utilization rate of valuable elements, and large sludge production , to achieve the effect of shortening the wastewater treatment process, reducing the amount of sludge produced, and reducing the rate of sludge production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
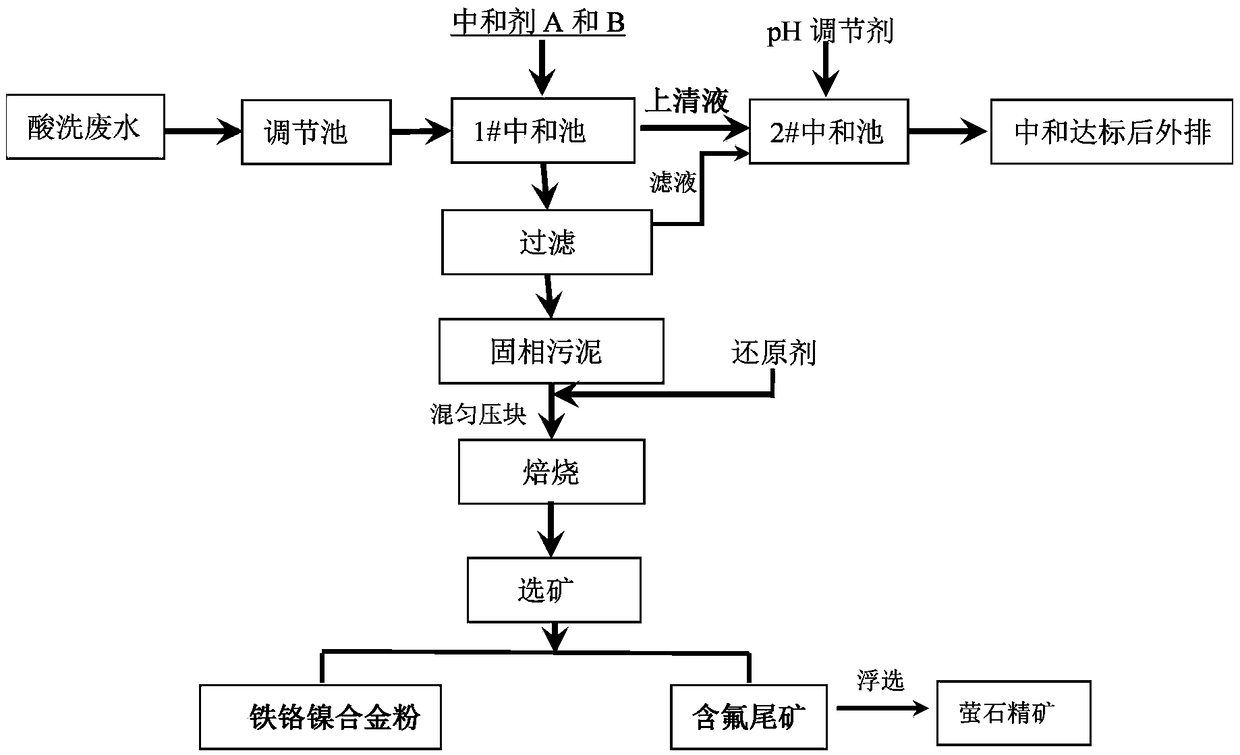
[0036] Such as figure 1 Shown, a kind of stainless steel pickling waste water resource recovery method, described method comprises the following steps:
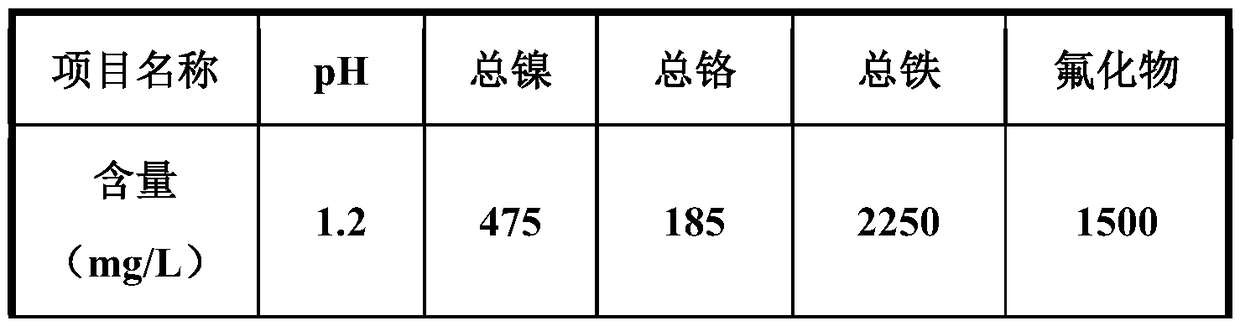
[0037] a. Take 500mL of acid wastewater with pH=1.2, first add CaO to adjust the pH to 4.0, the amount of CaO added is 3.6g, then add NaOH to adjust the pH to 9.0, the amount of NaOH added is 1.5g, and the mass ratio of CaO to NaOH is 2.4 , fully stirred and reacted in 1# neutralization tank for 1h;
[0038] b. After fully reacting, the liquid in the 1# neutralization tank is put into the 1# settling tank for sedimentation, the supernatant is put into the 2# neutralization tank, the solid phase sludge is filtered, and the filter residue is dried to obtain dry sludge 6.74 g, the filtrate returns to the 2# neutralization pool;
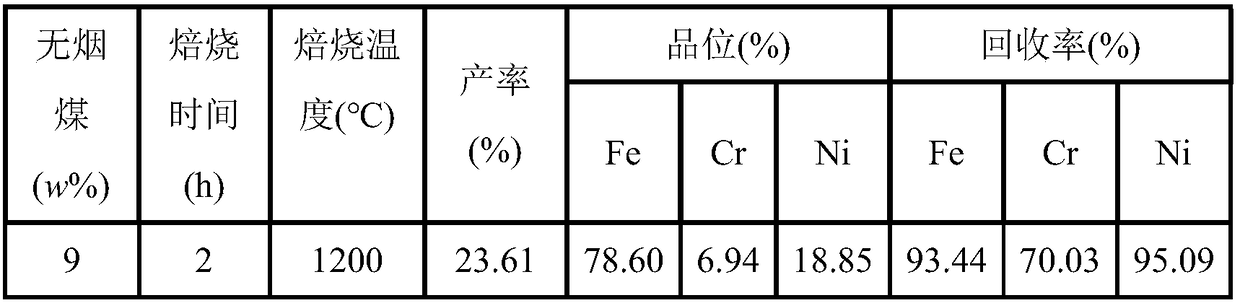
[0039] c. The dried filter residue is mixed evenly with anthracite, the amount of anthracite added is 9% of the filter residue, the briquette is placed in a graphite crucible, and roasted at 1200 ° C ...
Embodiment 2
[0044] Such as figure 1 Shown, a kind of stainless steel pickling waste water resource recovery method, described method comprises the following steps:
[0045] a. Take 500mL acid wastewater with pH=1.2, add Ca(OH) first 2 Adjust pH to 4.0, CaO addition is 5.4g, add NaOH to adjust pH to 9.0, NaOH addition is 1.5g, Ca(OH) 2 The mass ratio to NaOH is 3.6, fully stirred and reacted in 1# neutralization tank for 1h;
[0046]b. After fully reacting, the liquid in the 1# neutralization tank is put into the 1# settling tank for sedimentation, the supernatant is put into the 2# neutralization tank, the solid phase sludge is filtered, and the filter residue is dried to obtain dry sludge 6.84 g, the filtrate returns to the 2# neutralization pool;
[0047] c. The dried filter residue is mixed evenly with anthracite, the amount of anthracite added is 9% of the filter residue, the briquette is placed in a graphite crucible, and roasted at 1200 ° C for 2 hours;
[0048] d. After roastin...
Embodiment 3
[0055] Such as figure 1 Shown, a kind of stainless steel pickling waste water resource recovery method, described method comprises the following steps:
[0056] a. Take 500mL of acid wastewater with pH=1.2, first add CaO to adjust the pH to 4.0, the amount of CaO added is 3.6g, then add NaOH to adjust the pH to 12.0, the amount of NaOH added is 3.2g, and the mass ratio of CaO to NaOH is 1.13 , fully stirred and reacted in 1# neutralization tank for 1h;
[0057] b. After fully reacting, the liquid in the 1# neutralization tank is put into the 1# settling tank for sedimentation, the supernatant is put into the 2# neutralization tank, the solid phase sludge is filtered, and the filter residue is dried to obtain dry sludge 6.74 g, the filtrate returns to the 2# neutralization pool;
[0058] c. The dried filter residue is mixed evenly with anthracite, the amount of anthracite added is 12% of the filter residue, the briquetting is placed in a graphite crucible, and roasted at 1200...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com