Sulfonamide compounds as well as preparation method and application thereof
A technology for sulfonamides and compounds, which is applied in the field of sulfonamides and their preparation, and can solve problems such as unfavorable applications and complex synthesis steps of sulfonamides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Embodiment one 2-((triisopropylsilyl) acetylene) benzenesulfonamide (3a)
[0104]
[0105] Under nitrogen atmosphere, add arylsulfonamide compound 1a (14.4mg, 0.10mmol) and alkynylation reagent 2 (28μL, 0.20mmol) to a 15mL Schlenk reaction tube sequentially, when X=Br or I, no additional oxidant is needed; When X=H, AgOAc(2equiv.);), dichloro(pentamethylcyclopentadienyl)iridium dimer (2.3 mg, 0.0025 mmol) or dichloro(p-cymene) are required Ruthenium dimer (1.5mg, 0.0025mmol), silver bis(trifluoromethanesulfonyl)imide (4.2mg, 0.015mmol) or silver hexafluoroantimonate (5.2mg, 0.015mmol), cesium acetate (30mg, 0.36mmol), 1,2-dichloroethane (DCE, 1mL), react at 120°C for 12 hours. After the reaction was completed, it was cooled to room temperature, filtered through diatomaceous earth, and concentrated to obtain a crude product. The crude product was separated by chromatography on a prepared silica gel plate, and the selected developer or eluent was petroleum ether and ...
Embodiment 2
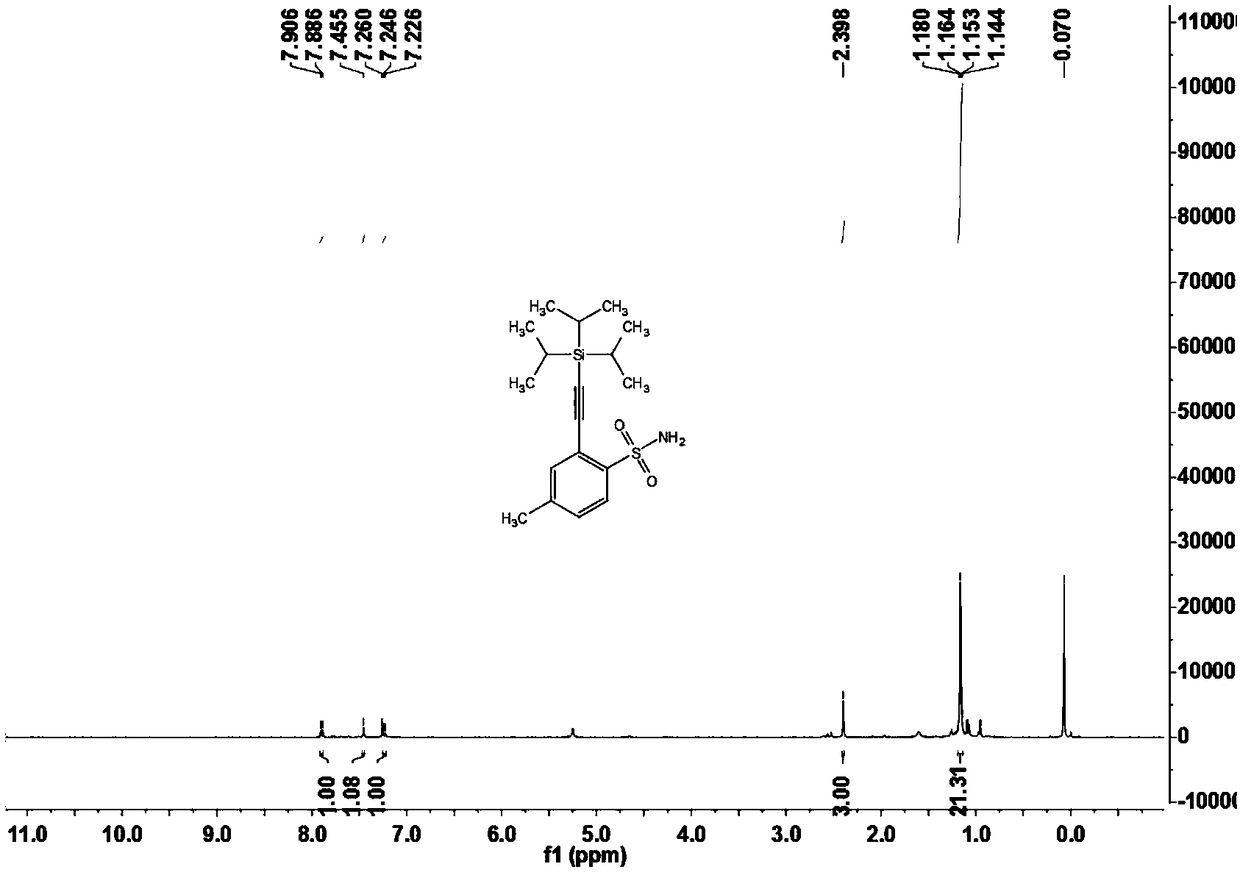
[0108] Example two 4-methyl-2-((triisopropylsilyl)acetylene)benzenesulfonamide (3b)
[0109]
[0110] Under nitrogen atmosphere, add arylsulfonamide compound 1b (17.1mg, 0.10mmol) and alkynylation reagent 2 (20μL, 0.15mmol) to a 15mL Schlenk reaction tube sequentially, when X=Br or I, no additional oxidant is needed; When X=H, AgOAc (2equiv.);), dichloro(pentamethylcyclopentadienyl) iridium dimer (2.3mg, 0.0025mmol) or dichloro(p-cymene) Ruthenium dimer (1.5mg, 0.0025mmol), silver bis(trifluoromethanesulfonyl)imide (4.2mg, 0.015mmol) or silver hexafluoroantimonate (5.2mg, 0.015mmol), cesium acetate (30mg, 0.36mmol), 1,2-dichloroethane (DCE, 1mL), react at 120°C for 12 hours. After the reaction was completed, it was cooled to room temperature, filtered through diatomaceous earth, and concentrated to obtain a crude product. The crude product was chromatographically separated on a prepared silica gel plate, and the selected developer or eluent was petroleum ether and ethyl a...
Embodiment 3
[0113] Example 3 4-((2Z,3E)1-methyl-3-phenyl-2-propenylhydrazone)-2-((triisopropylsilyl)acetylene)benzenesulfonamide (3c)
[0114]
[0115] Under nitrogen atmosphere, add sulfonamide compound 1c (31.5 mg, 0.10 mmol) and alkynylation reagent 2 (20 μ L, 0.15 mmol) sequentially to a 15 mL Schlenk reaction tube, when X=Br or I, no additional oxidant is needed; when X =H, need AgOAc (2equiv.);), dichloro(pentamethylcyclopentadienyl)iridium dimer (2.3mg, 0.0025mmol) or dichloro(p-methylcymenyl)ruthenium bis polymer (1.5mg, 0.0025mmol), silver bis(trifluoromethanesulfonyl)imide (4.2mg, 0.015mmol) or silver hexafluoroantimonate (5.2mg, 0.015mmol), cesium acetate (30mg, 0.36mmol ), 1,2-dichloroethane (DCE, 1 mL), reacted at 120°C for 18 hours. After the reaction was completed, it was cooled to room temperature, filtered through diatomaceous earth, and concentrated to obtain a crude product. The crude product was separated by chromatography on a prepared silica gel plate, and the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com