A class of a with thiazolothiazole as the core 2 -π-a 1 -π-a 2 Preparation and Application of Small Molecule Acceptor Materials
A small molecule acceptor, thiazolo technology, applied in the application of electron acceptor in the field of organic photovoltaic cells, in the field of preparation of A2-π-A1-π-A2 type small molecule acceptor materials, which can solve the problem of film formation stability Difficulty in performance adjustment and high synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] A with thiazolothiazole as the nucleus 2 -π-A 1 -π-A 2 Synthesis of small molecule receptor material TTz1.
[0050] The synthetic route of TTz1 is as follows:
[0051]
[0052] 1. Synthesis of 12-bromo-3-(2-butyloctyl)thiophene (2)
[0053] In a 250 mL three-necked flask, sequentially add compound 1 (10 g, 39.6 mmol), N-bromosuccinimide (8.46 g, 47.6 mmol), and 150 mL of glacial acetic acid. Under the protection of light, react at room temperature for 12 hours, stop the reaction, transfer the reaction solution to a separatory funnel, add 50mL distilled water, extract with dichloromethane, dry over anhydrous magnesium sulfate, distill the organic solvent under reduced pressure, and the residue is distilled with petroleum ether Column chromatography was used as the eluent to obtain compound 2 as a colorless oil (12.2 g, yield 93%). 1 H NMR (400MHz, CDCl 3 )δ7.18(d, J=5.6Hz, 1H), 6.76(d, J=5.6Hz, 1H), 2.49(d, J=7.2Hz, 2H), 1.65(dd, J=11.1, 5.9Hz, 1H), 1.29-1.24 (...
Embodiment 2
[0066] A with thiazolothiazole as the nucleus 2 -π-A 1 -π-A 2 Synthesis of small molecule receptor material TTz2.
[0067] The synthetic route of TTz2 is as follows:
[0068]
[0069] In a 100mL two-neck flask, sequentially add compound 7 (200mg, 0.21mmol), compound 8 (382mg, 2.07mmol), triethylamine (0.5mL) and chloroform (60mL), under the protection of nitrogen, reflux reaction at 65°C 24h. Cool to room temperature, pour into 200 mL of anhydrous methanol for precipitation, filter with suction, and use chloroform as the eluent to separate the crude product by column chromatography, recrystallize from chloroform and acetone to obtain a maroon solid with metallic luster Compound TTz2 (230 mg, 83% yield). 1 H NMR (400MHz, CDCl 3 )δ8.32(s,1H),7.95(s,1H),7.76(d,J=7.7Hz,1H),7.56(d,J=7.7Hz,1H),4.35-4.29 (m,2H), 2.94-2.82 (m, 2H), 1.89 (t, J=16.9Hz, 1H), 1.51-1.26 (m, 19H), 0.97-0.87 (m, 6H).
Embodiment 3
[0071] Performance characterization of small molecule acceptor materials TTz1 and TTz2 and preparation and testing of photovoltaic devices:
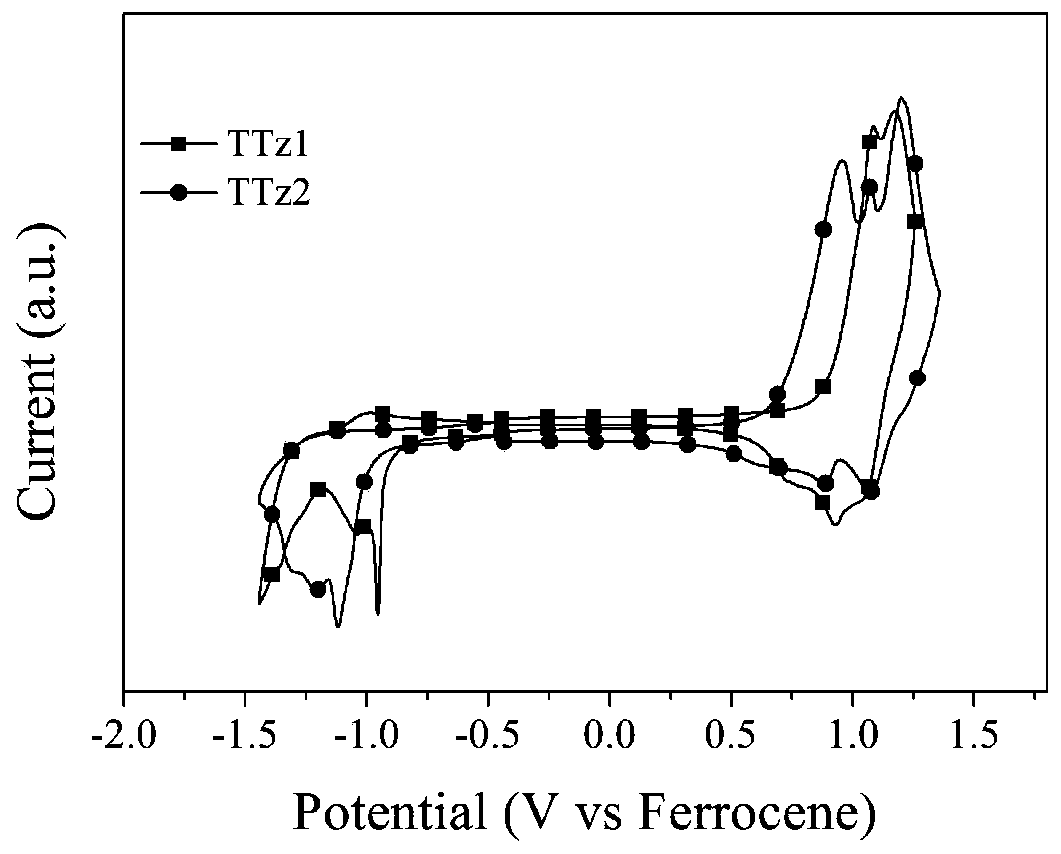
[0072] of the synthesized compound 1 The H NMR spectrum was measured by Bruker Dex-300 NMR or 400NMR instrument, the UV-visible absorption spectrum was measured by Shimadzu UV-800 UV-visible spectrophotometer, and the cyclic voltammetry curve was tested by CHI630E electrochemical analyzer.
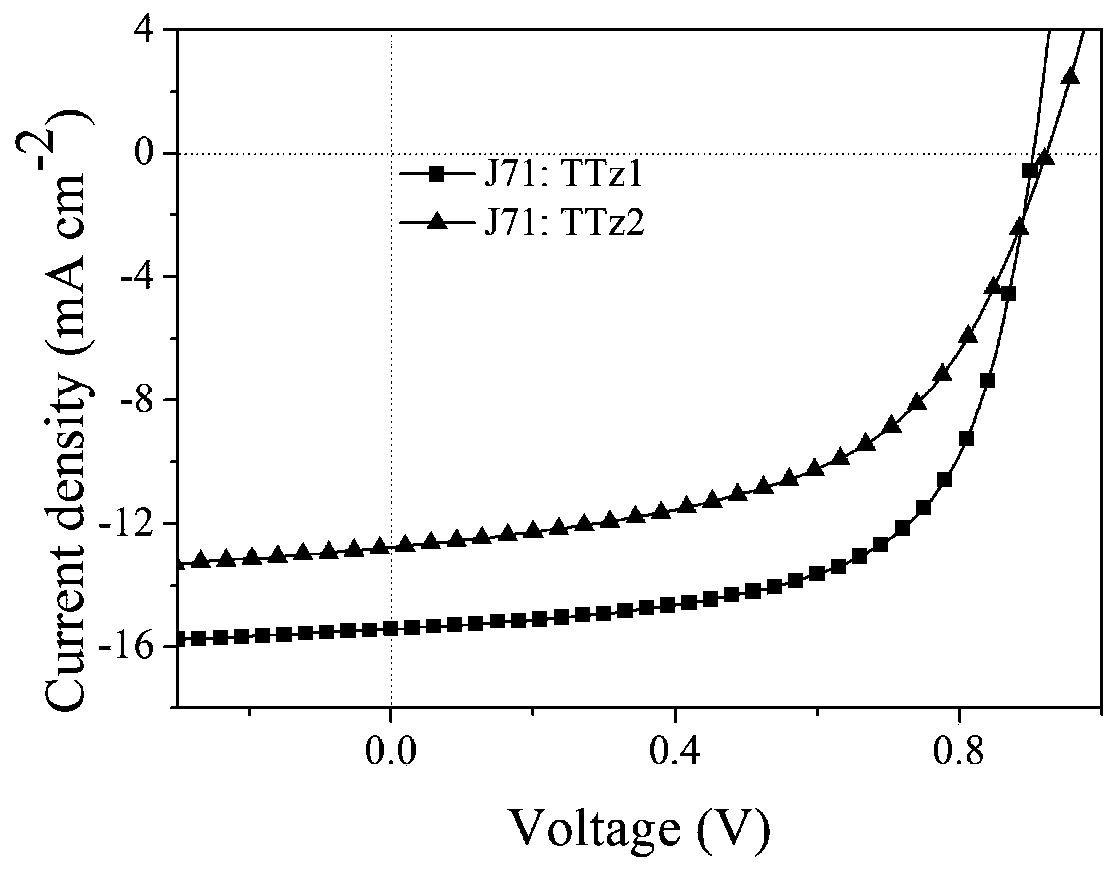
[0073] An organic solar cell device based on this type of small molecule acceptor material includes an indium tin oxide (ITO) conductive glass anode, a cathode modification layer, a photoactive layer, an anode modification layer, and a cathode. The active layer material is the small molecule acceptor material described in the present invention and a commercially purchased polymer donor material, and the blending weight ratio is 1:2.
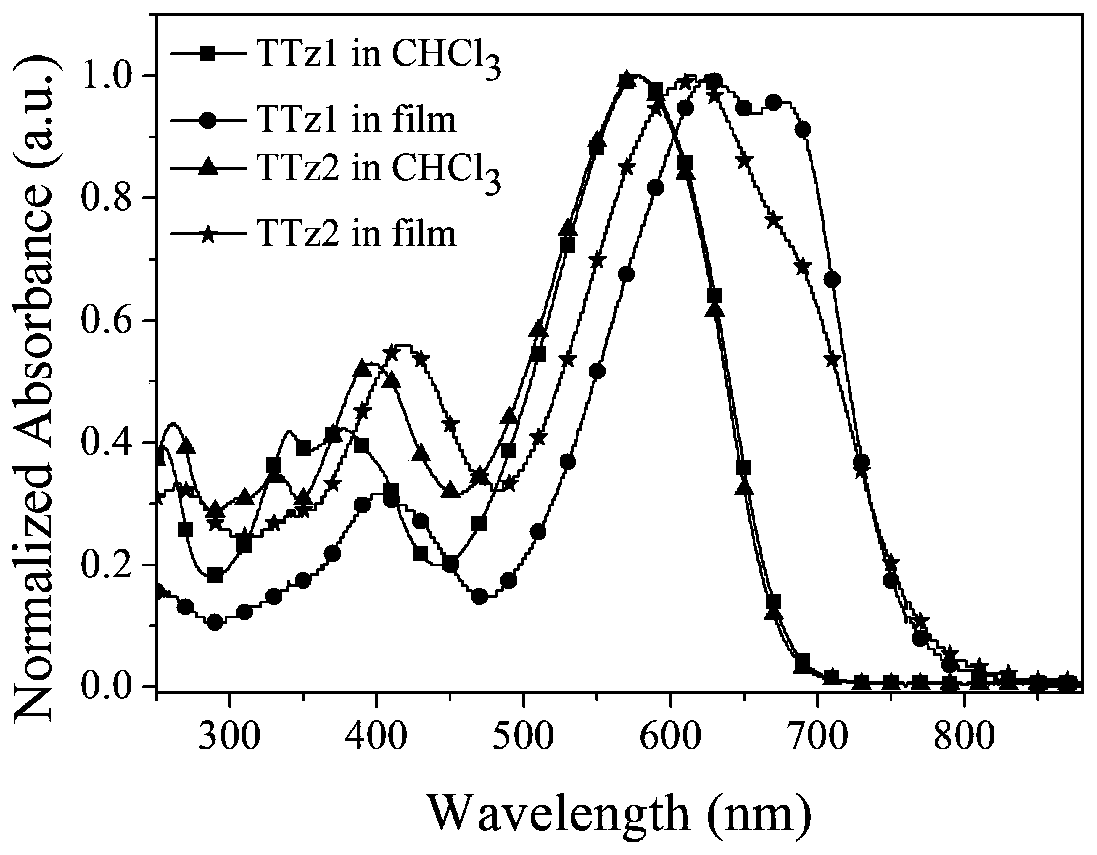
[0074] 3.1 Determination of photophysical properties of small organic molecule receptors TTz1 and TTz2
[0075] fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical band gap | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com