Preparation method of 2-pyrrolyl-1,3-oxazacyclohexane compound
A technology for oxazines and compounds, which is applied in the field of organic chemical synthesis, can solve the problems of strong toxicity, difficult reaction connection of alkane long-chain structures, low product yield, etc., and achieves the effect of optimizing reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] (1) Knoevenagel condensation reaction
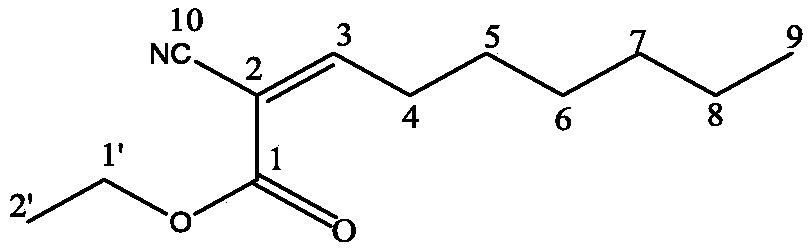
[0065] Add 0.1 mol of n-heptanal to the solvent dichloromethane, mix well; slowly add 0.1 mol of ethyl cyanoacetate and 0.25 g of sodium ethoxide solid catalyst under the condition of stirring at 20°C, then gradually raise the temperature to 35°C for reflux reaction for 4 hours, TLC tracking Reaction process; after the reaction, cool the reaction product to 20°C, filter to remove the precipitate, and obtain a mixture of 2-cyano-2-nonenoic acid-ethyl ester and unreacted raw materials; then add an equal volume of saturated salt to the mixture Water extraction, extraction twice, combined organic phases, dried to remove water, evaporated solvent to obtain 2-cyano-2-nonenoic acid-ethyl ester; yield 53%;
[0066] (2) Reduction reaction
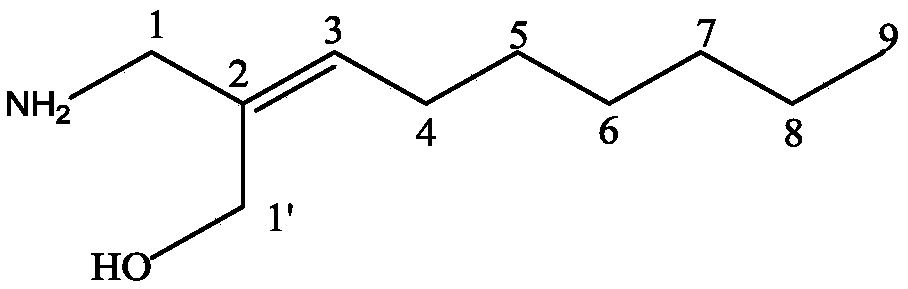
[0067] The 2-cyano-2-nonenoic acid-ethyl ester prepared in step (1) and 0.4g lithium aluminum hydride were respectively dissolved in the solvent anhydrous ether to obtain 2-cyano-2-nonenoic acid-ethyl ...
Embodiment 2
[0071] (1) Knoevenagel condensation reaction
[0072] Add 0.2 mol of n-heptanal to the solvent dichloromethane, mix well; slowly add 0.2 mol of ethyl cyanoacetate and 0.35 g of sodium ethoxide solid catalyst under the condition of stirring at 25°C, then gradually raise the temperature to 40°C for reflux reaction for 4.5h, TLC Track the reaction process; after the reaction, cool the reaction product to 25°C, filter to remove the precipitate, and obtain a mixture of 2-cyano-2-nonenoic acid-ethyl ester and unreacted raw materials; then add an equal volume of saturated Extract with salt water, extract 3 times, take the organic phases and combine them, remove the water after drying, and evaporate the solvent to obtain 2-cyano-2-nonenoic acid-ethyl ester; the yield is 50%;
[0073] (2) Reduction reaction
[0074] The 2-cyano-2-nonenoic acid-ethyl ester prepared in step (1) and 0.5g lithium aluminum hydride were respectively dissolved in the solvent anhydrous ether to obtain 2-cyano...
Embodiment 3
[0078] (1) Knoevenagel condensation reaction
[0079] Add 0.3 mol of n-heptanal to the solvent dichloromethane, mix well; slowly add 0.3 mol of ethyl cyanoacetate and 0.45 g of sodium ethoxide solid catalyst under the condition of stirring at 30°C, then gradually raise the temperature to 50°C for reflux reaction for 5 hours, TLC tracking Reaction process; after the reaction, cool the reaction product to 30°C, filter to remove the precipitate, and obtain a mixture of 2-cyano-2-nonenoic acid-ethyl ester and unreacted raw materials; then add an equal volume of saturated salt to the mixture Extracted with water, extracted 3 times, combined the organic phases, dried to remove water, and evaporated the solvent to obtain 2-cyano-2-nonenoic acid-ethyl ester; the yield was 49%;
[0080] (2) Reduction reaction
[0081] The 2-cyano-2-nonenoic acid-ethyl ester prepared in step (1) and 0.6g lithium aluminum hydride were respectively dissolved in the solvent anhydrous ether to obtain 2-cya...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com