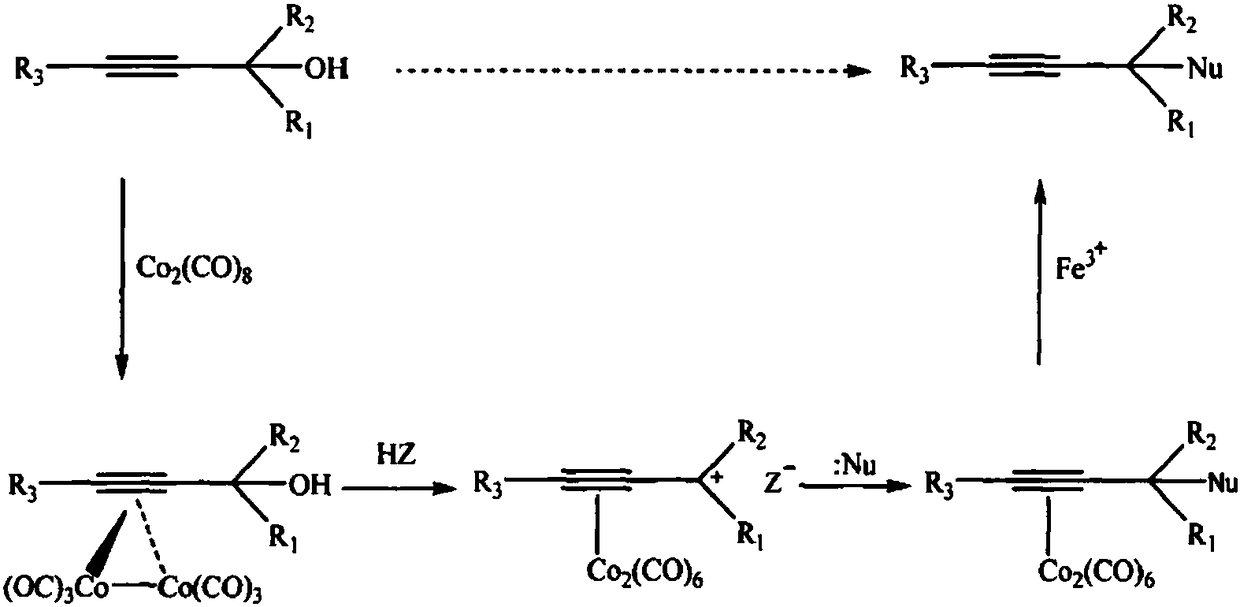
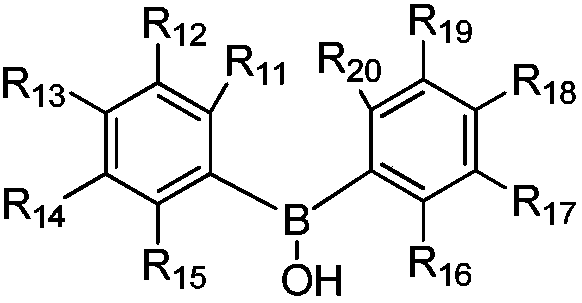
Composition for preparing propargyl ether
A technology of propargyl ether and composition, applied in the field of preparing propargyl ether composition, can solve problems such as high toxicity, increased reaction cost, operator injury and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Embodiment 1 The preparation method of propargyl ether of the present invention
[0133]
[0134] In a nitrogen-filled glove box, the Et 3 N (56 μL, 0.40 mmol, 2.0 equiv), boric acid B1 (2.0 mg, 0.010 mmol, 5.0 mol%), fatty alcohol (0.24 mmol, 1.2 equiv) and THF (0.3 mL) were sequentially added to a screw cap vial (vial A) . Cap vial A tightly and take it out of the glove box.
[0135] In a nitrogen-filled glove box, the Cu(CH 3 EN) 4 BF 4 (3.2mg, 0.010mmol, 5.0mol%), Ligand L5 (4.8mg, 0.020mmol, 10mol%) and THF (0.2mL) were sequentially added to another screw cap vial (vial B) with a magnetic stir bar . Vial B was stirred at 60 °C for 1 h, cooled to room temperature, and propargyl ester (0.20 mmol, 1.0 equiv) was added. After the vial B was tightly capped, it was taken out of the glove box, stirred at room temperature for 10 min, and cooled to -20°C. At this temperature, the solution in vial A was added to vial B via an airtight syringe, and the resulting re...
Embodiment 2
[0137] Example 2 The universality of the composition of the present invention for the preparation of propargyl ether
[0138]
[0139] a, the universality of propargyl compounds (with the above-mentioned compound 11 as fatty alcohol substrate):
[0140]
[0141] b, the universality of fatty alcohols (with the above-mentioned compound 10 as the propargyl substrate):
[0142]
[0143] Note: Unless otherwise specified, the reactions in the examples were carried out on a 0.2 mmol scale, Cu(CH 3 EN) 4 BF 4 (5mol%), L5 (10mol%), B1 (5mol%), Et 3 N(2equiv), reacted at -20°C for 24h. The yield is the separation yield, the ee value is detected by HPLC, and the dr value is detected by the crude NMR of the reaction solution 1 H NMR determination. [a] Ligand L6 is used.
[0144] Above-mentioned reaction result proves the universality of propargyl ether preparation method of the present invention: 1, with respect to propargyl base substrate, aromatic ring is (10a-b, 10g) of...
Embodiment 3
[0145] Example 3 The composition of the present invention is used for the regioselective propargylation of carbohydrate derivatives
[0146] Sugar is an extremely important class of chemical structural units. Sugar is not only a necessary group for the function of glycoproteins and glycolipids, but also an important component of many natural drug molecules. For a long time, the research on sugars has provided many means for human beings to understand nature, and has also produced many important marketed drugs.
[0147] The selective introduction of terminal alkynes into sugar compounds will provide great convenience for sugar research. Because, the terminal alkyne is a functional group that can participate in "click chemistry". The role of "click chemistry" in the field of sugar research has long been confirmed. At the same time, the terminal alkyne itself is also a functional group that can be easily derivatized, and can easily synthesize various derivatives of sugars. In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com