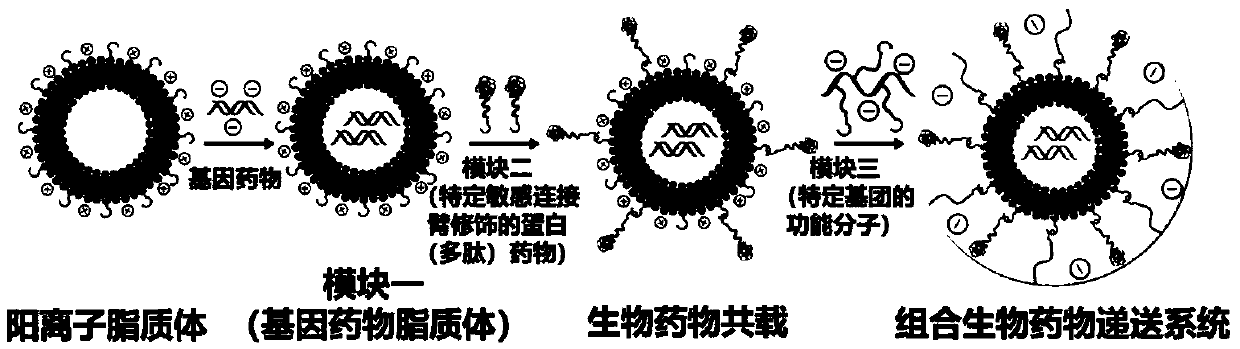
Modularly-assembled combined biological drug joint delivery system and application
A delivery system and modular technology, applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as inability to obtain technical inspiration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Preparation and Characterization of Azadibenzocyclooctyne Modified Reduction Sensitive Blank Cationic Liposomes (ADIBO-CL)
[0102] The reduction-sensitive blank cationic liposomes were prepared by thin film dispersion method. Weigh 25 mg of soybean lecithin, 2-(4-(2-(2-(2-(2-(2,6-diamino-hexylamino)-3-(1 hydrogen-imidazolyl-4-yl) isopropyl Amino)ethyl)dithio)diethylamino)-4-oxobutanone)tetradecyl glutarate (LHSSG2C 14 ) 5mg, azadibenzocyclooctyne (ADIBO) modified cholesterol 6mg and azadibenzocyclooctyne (ADIBO) modified DSPE 4mg, dissolved in chloroform / methanol mixed solvent, evaporated under reduced pressure to form a film, and Dry in vacuo overnight to remove residual organic solvent. Hydrate the lipid film at room temperature, disperse the obtained liposome suspension with an ultrasonic cell pulverizer, and pass through a 0.22 μm microporous membrane to obtain a blank liposome solution. Its particle size and potential properties are shown in Table 1.
[0103] ...
Embodiment 2
[0106] Preparation of Gene Drug Liposomes (AD-RSC) with Different Nitrogen-Phosphorus Ratio in Module 1
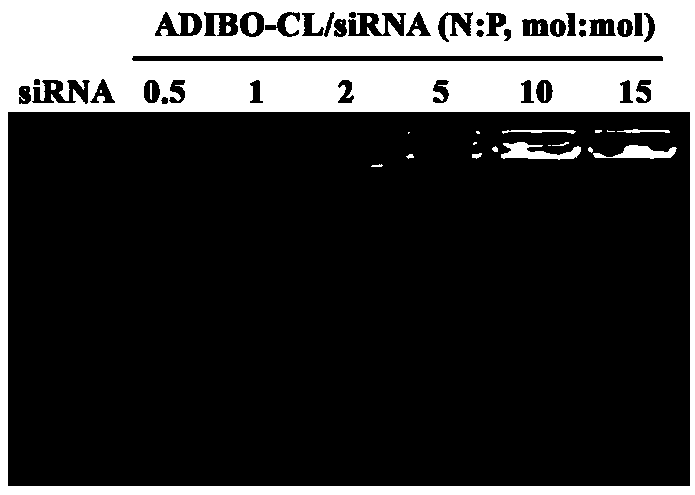
[0107] After diluting ADIBO-CL 5-10 times with pH 7.4 buffer, add different N / P (0.5, 1.0, 2.0, 5.0, 10, 15) siRNA (sense strand: 5'-CUUGUGCUCAAACUCGUCCdTdT-3', Sense strand: 3'-dTdTGAACACGAGUUUGAGCAGG-5") mixed and allowed to stand at room temperature for 30min to obtain the gene drug liposome (Module 1, AD-RSC). The loading of cationic liposome on siRNA was investigated by agarose gel electrophoresis ability, the results show that when N / P is greater than 5, cationic liposomes can fully load siRNA see image 3 . The particle size and potential of AD-RSC were measured by dynamic light scattering method. The experimental results showed that with the increase of N / P, the particle size of AD-RSC first increased and then decreased, and its surface potential changed from negative to positive. See Figure 4 .
Embodiment 3
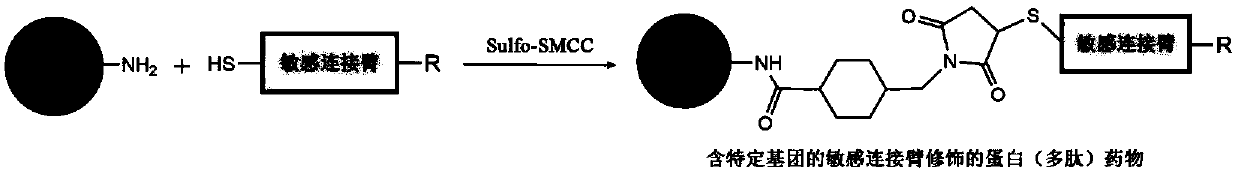
[0109] Modified protein (polypeptide) drug (N 3 -s-TRAIL) preparation
[0110] Using Sulfo-SMCC as the coupling agent between the protein (polypeptide) drug and the sensitive linker, mix 1.0mg / mL, 5mL TRAIL with 1.0mg / mL, 1mL azide-enzyme-sensitive linker or acid-sensitive linker (specifically The structure is as follows) were dissolved in PBS buffer, and then 4.8mg / mL, 500μL Sulfo-SMCC was added and reacted at room temperature for 1h. After the reaction was completed, the reaction solution was dialyzed at room temperature for 3 days to obtain the TRAIL modified with the azide-enzyme-sensitive linker.
[0111] The enzyme-sensitive linker is: N 3 -Pro-Leu-Gly-Leu-Ala-Gly-Cys-SH
[0112] The acid-sensitive linking arm is: N 3 -CH 2 -HN-N=CH-CH 2 -SH
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com