Fluorescently labeled polysaccharide as well as preparation method and application thereof
A fluorescent labeling and polysaccharide technology, applied in the field of medical biological detection, can solve the problems of unstable fluorescent signal, inability to effectively distinguish different fluorescent signals, small Stokes shift, etc., to increase the variety of probes, low toxicity, and selectivity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
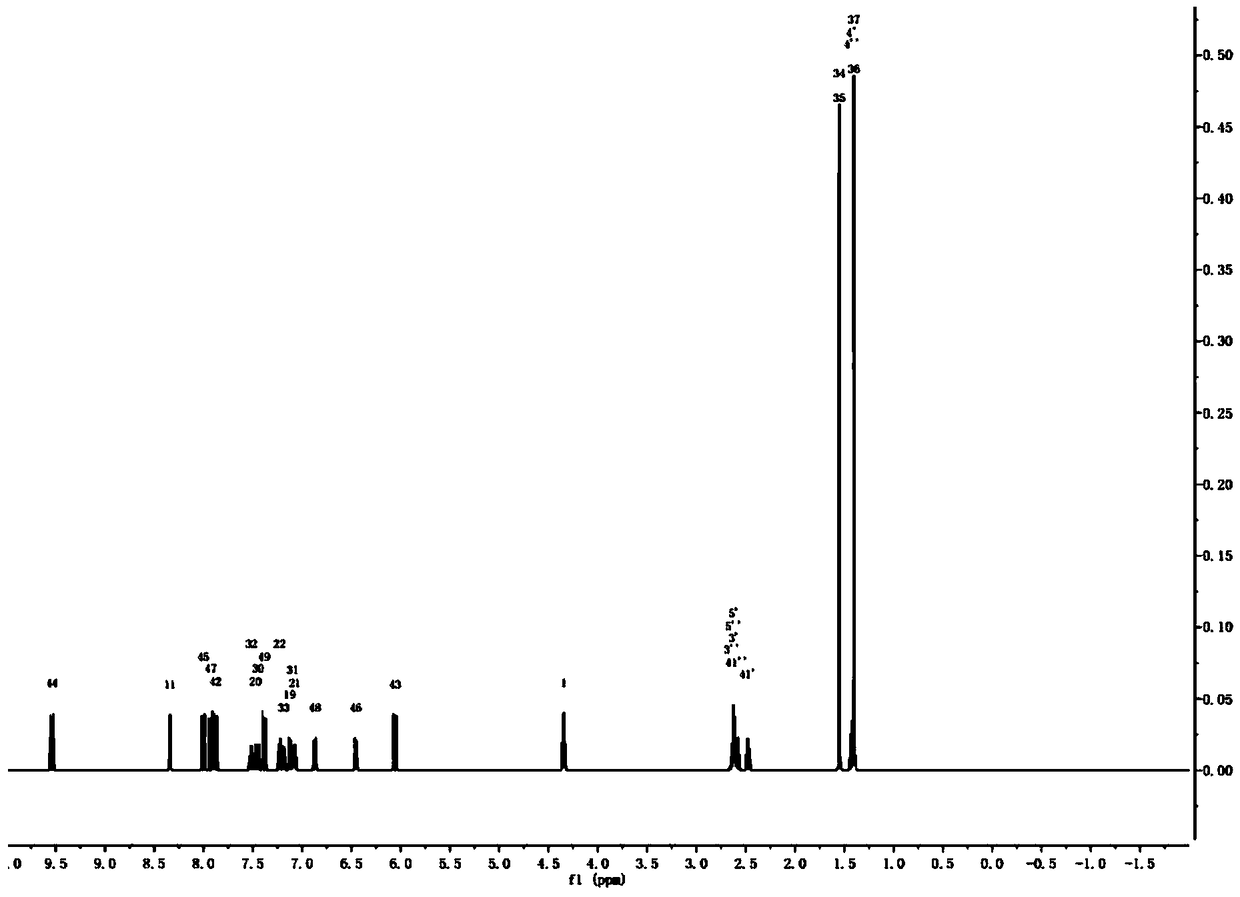
[0053] This embodiment provides a fluorescent dye having a structure represented by the following formula B:
[0054]
[0055] The fluorescence synthesis route shown in formula B is as follows:
[0056]
[0057] (1) Preparation of Intermediate I’-1
[0058] Add phenylhydrazine to glacial acetic acid, stir, slowly add 3-methyl-2-butanone dropwise, heat to 62.5°C after dropwise addition, react for 3-4 hours, extract, concentrate, and refine to obtain intermediate I'- 1;
[0059] Among them, the molar ratio of phenylhydrazine and 3-methyl-2-butanone is 1:1.1;
[0060] (2) Preparation of Intermediate I’-2
[0061] Add Intermediate I'-1 and 1,2-Dibromoethylene into toluene, protected by nitrogen, heated and refluxed for 17 hours, cooled, and precipitated a solid, namely Intermediate I'-2;
[0062] Among them, the molar ratio of intermediate Ⅰ-1 and 1,2-dibromoethylene is 1:1.75;
[0063] (3) Preparation of Intermediate I’-4
[0064] Add dry N,N-dimethylformamide to dry dichloromethane, add phos...
Embodiment 2
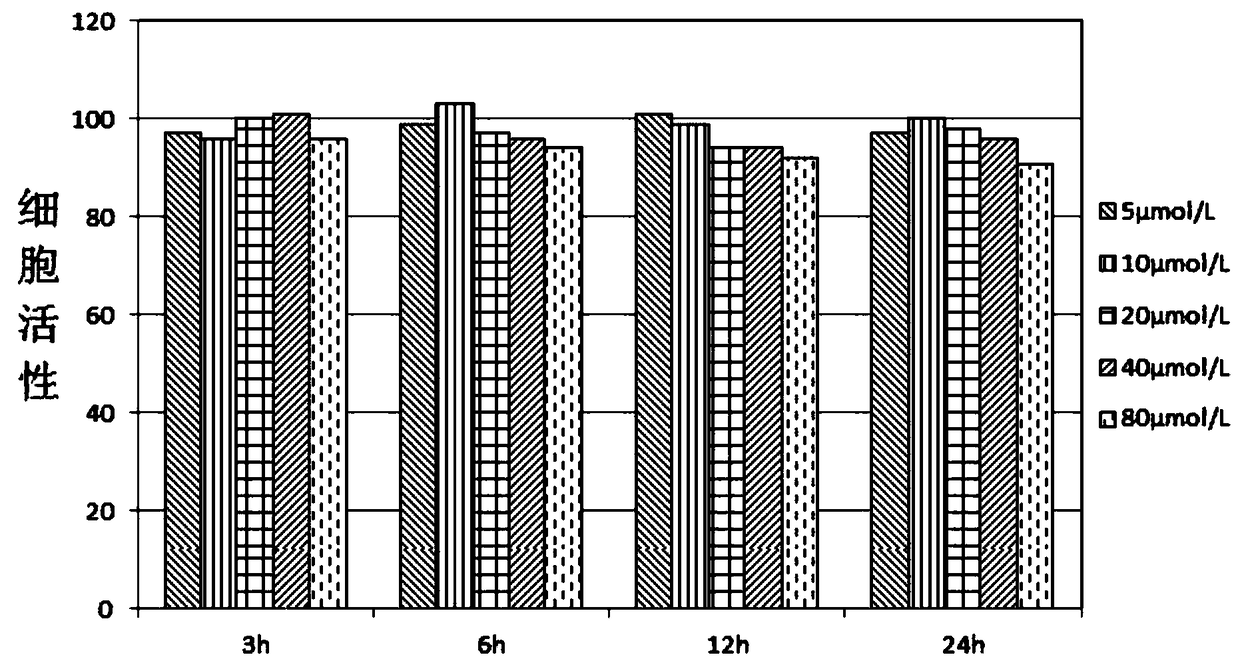
[0075] This embodiment provides a fluorescently labeled polysaccharide, wherein the polysaccharide is dextran (purchased from sigma, catalog number 00268), and the molecular structure of the fluorescent dye is as follows:
[0076]
[0077] The preparation method of fluorescently labeled polysaccharide includes the following steps:
[0078] 1. Prepare saturated cyanogen bromide solution and 0.2M NaOH solution;
[0079] 2. Weigh 20mg of dextran, dissolve the dextran in 1ml of distilled water, fully stir to dissolve; add 4ml of cyanogen bromide solution, add 25μI of NaOH solution each time, continue to add (ensure pH> 10) Continue for 7 minutes and observe the change of the solution. If precipitation occurs, the experiment will fail and repeat;
[0080] 3. Dialysis the reaction solution obtained in the previous step against distilled water, dialysis for 1-2 days, replace the dialysis external fluid every 2-3h; concentrate the solution in the bag to dryness, and add 0.2M Na with pH=8 2 B 4...
Embodiment 3
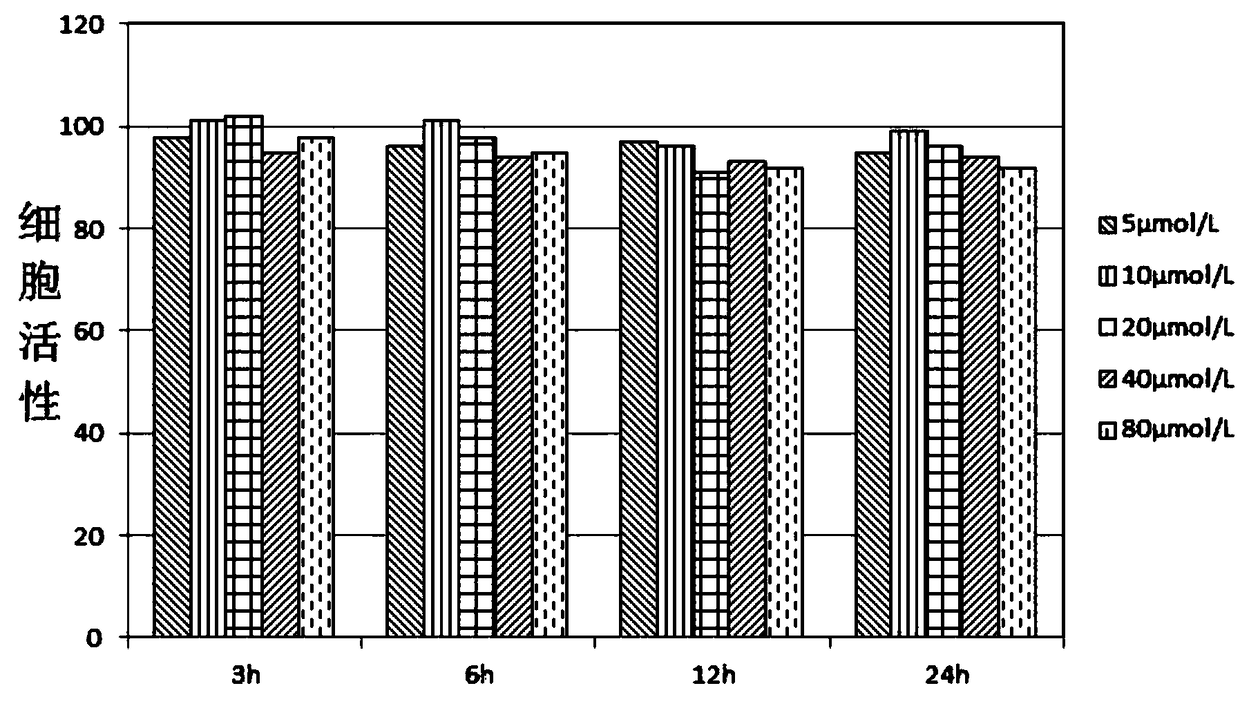
[0083] This embodiment provides a fluorescently labeled polysaccharide, wherein the polysaccharide is chitosan (purchased from sigma, catalog number 448869), and the molecular structure of the fluorescent dye is as follows:
[0084]
[0085] The preparation method of fluorescently labeled polysaccharide includes the following steps:
[0086] 1. Prepare saturated cyanogen bromide solution and 0.2M NaOH solution;
[0087] 2. Weigh 10mg of chitosan, dissolve the chitosan in 1ml of distilled water, fully stir to dissolve; add 4ml of cyanogen bromide solution, add 25μI of NaOH solution each time, add continuously (ensure pH> 10) Continue for 9 minutes and observe the change of the solution. If precipitation occurs, the experiment will fail and repeat;
[0088] 3. Dialysis the reaction solution obtained in the previous step against distilled water, dialysis for 1-2 days, replace the dialysis external fluid every 2-3h; concentrate the solution in the bag to dryness, and add 0.2M Na with pH=8 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com