Paclitaxel and diazepinocarbazole compound combined pharmaceutical composition
A technology of diazepines and carbazoles, which is applied in the field of combined pharmaceutical compositions of paclitaxel and diazazocarbazoles, can solve problems such as the ineffectiveness of anticancer agents, and achieves reduction of clinical dosage, toxic and side effects Low, good anti-cancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
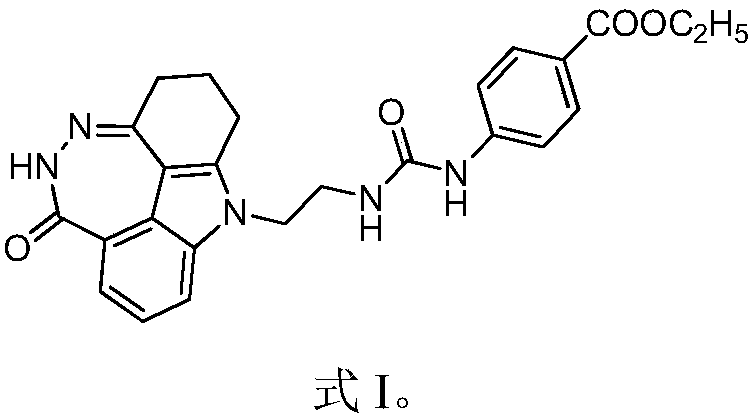
[0018] Example 1 1-(2,3,5,10-tetrahydro-[1,2]diazepine And[3,4,5,6-def]carbazol-6(1H)-one-10-yl)-3-(4-ethoxycarbonyl)phenylurea
[0019] Step 1 Synthesis of methyl 2-bromo-3-((3-oxocyclohex-1-en-1-yl)amino)benzoate
[0020]
[0021] Weigh 3.58g of methyl 3-amino-2-bromobenzoate and 1.68g of cyclohexane-1,3-dione into a reaction flask, add 20ml of acetic acid to dissolve, and react at 80°C for 8h. After the reaction, evaporate under reduced pressure Remove the solvent and purify by column chromatography to obtain the title compound. LC-MS m / z: [M+H]+=325.
[0022] Step 2 Synthesis of methyl 4-oxo-2,3,4,9-tetrahydro-1H-carbazole-5-carboxylate
[0023]
[0024] Weigh 1.62g of the product obtained in step 1, 0.23g of palladium acetate, 1.22g of tris(o-tolyl)phosphine and 0.63g of triethylamine in a sealed tube, add 15ml of acetonitrile, and seal the reaction at 100°C for 20 hours under a nitrogen atmosphere, and the reaction is complete After cooling, add 15ml of water ...
Embodiment 2
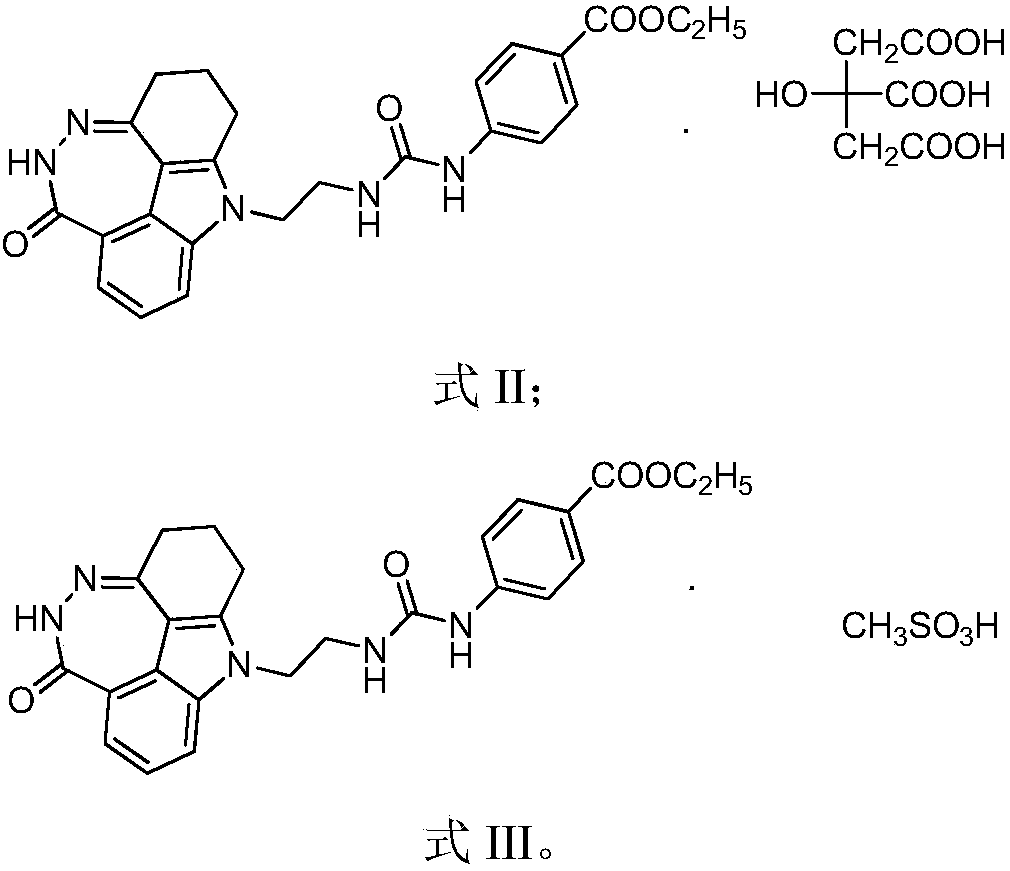
[0044] Example 2 1-(2,3,5,10-tetrahydro-[1,2]diazepine Synthesis of [3,4,5,6-def]carbazol-6(1H)-on-10-yl)-3-(4-ethoxycarbonyl)phenylurea citrate
[0045] Weigh 1.0 g of the compound of Example 1 into a reaction flask, add 20 ml of chloroform to dissolve it, add 0.42 g of citric acid, stir at room temperature for 3 h, and evaporate the solvent under reduced pressure to obtain the title compound.
Embodiment 3
[0046] Example 3 1-(2,3,5,10-tetrahydro-[1,2]diazepine Synthesis of [3,4,5,6-def]carbazol-6(1H)-on-10-yl)-3-(4-ethoxycarbonyl)phenylurea methanesulfonic acid
[0047] Weigh 2.0 g of the compound of Example 1 into a reaction flask, add 50 ml of dichloromethane to dissolve it, add 0.42 g of methanesulfonic acid, stir at room temperature for 1 h, evaporate the solvent under reduced pressure, and obtain the title compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com