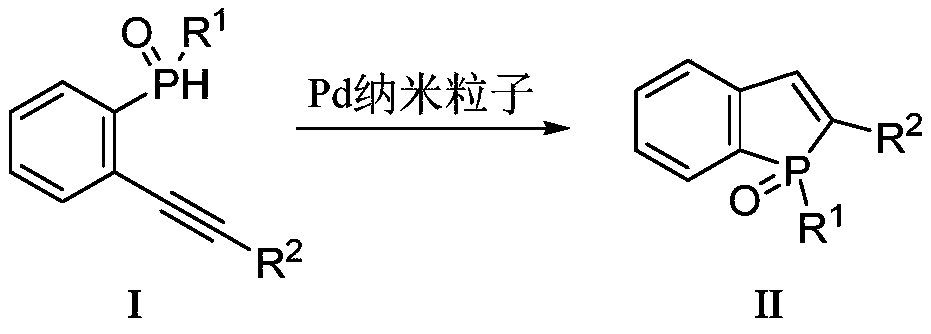
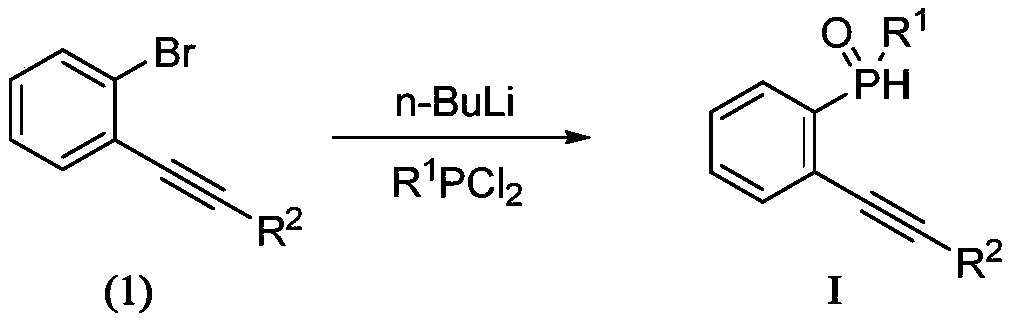
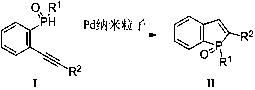A kind of method that catalyzes the synthesis of benzophosphole with pd nanoparticle
A technology of heterocyclopentadiene and benzophosphorus, which is applied in chemical instruments and methods, nanotechnology, catalyst activation/preparation, etc., and can solve problems such as complex reaction systems, cumbersome steps, and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: Preparation of Pd nanoparticles
[0017] First add 2ml of ethanol solvent to a 10ml reaction bottle, then add 5mmol of aniline and 12.5mmol of tetrafluoroboric acid, add 12.5mmol of tert-butyl nitrite dropwise to the mixed solution under ice bath conditions, return to room temperature, react for 0.5h, and proceed to the reaction A small amount of ether was added to the solution, and a white precipitate was formed, which was filtered and dried to obtain 0.92 g (4.8 mmol) of phenyldiazotetrafluoroborate, with a yield of 96%; 4 mmol of phenyldiazotetrafluoroborate was added to a 250ml reaction flask salt (dissolved in 50ml tetrahydrofuran), then added 1mmol palladium acetate (dissolved in 50ml methanol), stirred at room temperature for 10min to 15min, the reaction solution became orange, and then added dropwise 5mmol sodium borohydride (dissolved in 50ml methanol) The solution was added dropwise and reacted at room temperature for 1 h until the reaction was c...
Embodiment 2
[0018] Embodiment 2: the preparation of reaction substrate I
[0019]
[0020] Under nitrogen protection, add 2mmol of compound (1) to a 50ml reaction flask, then add 10ml of anhydrous diethyl ether and 10ml of anhydrous tetrahydrofuran, lower the temperature of the system to -78°C, add 2.1mmol of n-butyllithium in n-hexane dropwise under nitrogen Solution (1.6M), after the dropwise addition, keep it warm at -78°C for 1 hour (reaction system 1), take another reaction bottle, add 10ml of anhydrous ether and 2.2mmol of aryl phosphorus dichloride, and cool down to -78°C ( Reaction system 2), add the solution of reaction system 1 into reaction system 2, and keep it warm for 15 minutes, then return to room temperature and continue the reaction for 1 hour, and add 20ml of water to the system. Extracted with ethyl acetate and spin-dried to obtain a crude product, which was purified by column chromatography to obtain the corresponding solid compound I with a yield of 84%-92%.
Embodiment 3
[0021] Embodiment 3: Preparation of benzophosphole target compound II (example is not limited to this)
[0022]
[0023] Preparation of Compound Ⅱ—01
[0024] Add 1 mmol of compound I-01 (R 1 = Ph, R 2 =Ph), 3ml of toluene solvent, 3mg of the Pd nanoparticles prepared above, reacted at 30°C for 0.5h, TLC detected that the reaction was complete, after extraction and drying, the solvent was distilled off under reduced pressure, and then purified by column chromatography to obtain 287mg (0.95 mmol) Compound II-01, white solid, yield 95%.
[0025]
[0026] The analysis data is: 1 H NMR (400MHz, CDCl 3 )δ:7.29-7.54(m,10H),7.60-7.80(5H); 13 CNMR (100MHz, CDCl 3 )δ:124.70(d,J PC =9.6Hz), 126.62, 128.95(d, J PC =12.4Hz), 128.96(d, J PC =10.8Hz), 128.97, 129.23, 129.96 (d, J PC =97.8Hz), 130.75, 132.27, 132.54, 132.75 (d, J PC =108.3Hz), 133.26, 136.58, 138.74 (d, J CP =94.2Hz,C),141.64(d,J PC = 28.2Hz); 31 PNMR (162MHz, CDCl 3 ) δ=39.3.
[0027] Preparation of Co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com