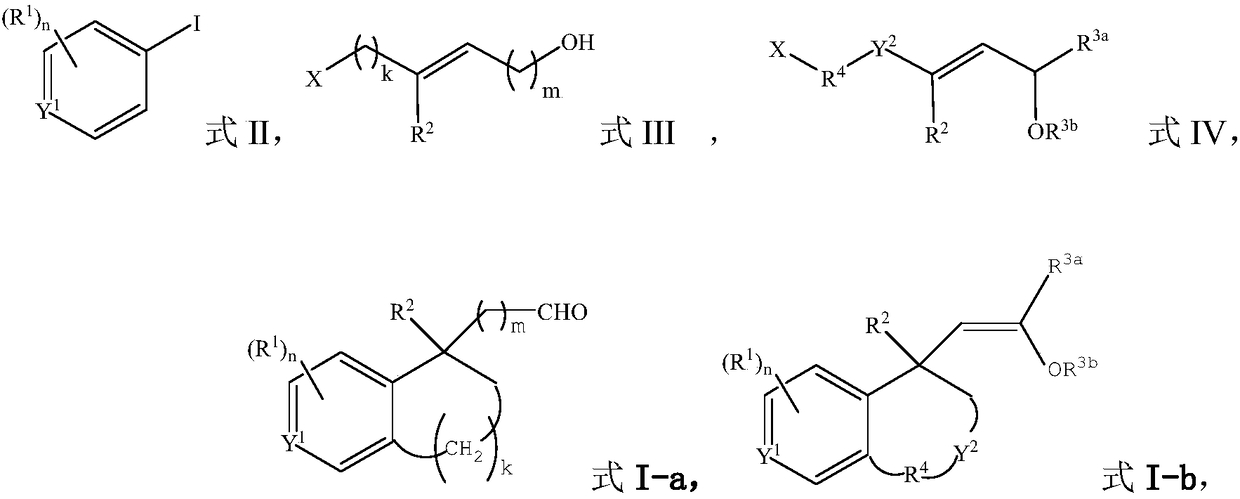
Methods for preparing benzo ring derivative with benzylic quaternary carbon center and ethazocine hydrobromide
A compound, heterocyclic aryl technology, applied in the field of benzo ring derivatives, can solve problems such as low efficiency and long synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the preparation of compound I-1
[0054]
[0055] Under the protection of argon gas, add catalyst allylpalladium chloride dimer (3.7mg, 0.01mmol), ligand XPhos (10.5mg, 0.022mmol), potassium carbonate to a dry reaction tube equipped with a magnetic stirrer (69.1 mg, 0.5 mmol) and dry acetonitrile (1.0 mL), the mixture was stirred at room temperature for 15 minutes. Dissolve aryl iodide [1-iodonaphthalene (61mg, 0.24mmol)], alkyl bromide [trans-6-bromo-3-methyl-2-hexen-1-ol (38.6mg, 0.2mmol )] and 5-norbornene-2-carboxylic acid (5.5mg, 0.04mmol) in dry acetonitrile (1.0mL) were added to the reaction tube, and then heated to 70°C for 24 hours under an argon atmosphere. After the reaction was cooled to room temperature, the mixture was filtered with celite, washed with ethyl acetate, the solvent was removed under reduced pressure, and purified by column chromatography to obtain compound I-1 (colorless oily liquid, yield 81%). 1 H NMR (400MHz, CDCl 3 ): ...
Embodiment 2
[0056] Embodiment 2: the preparation of compound 1-2
[0057]
[0058]The alkyl bromide used was trans 7-bromo-4-methyl-3-hepten-1-ol (41.4 mg, 0.2 mmol), and other conditions were the same as in Example 1 to obtain compound I-2 (colorless oily liquid, 68% yield). 1 H NMR (400MHz, CDCl 3 ): δ9.58(t, J=1.6Hz, 1H), 8.34(d, J=8.7Hz, 1H), 7.79(dd, J=8.0, 1.7Hz, 1H), 7.60(d, J=8.3Hz ,1H),7.46–7.36(m,2H),7.17(d,J=8.4Hz,1H),2.98–2.87(m,2H),2.78–2.71(m,1H),2.31–2.22(m,1H ),2.13–2.06(m,1H),1.95–1.81(m,4H),1.73–1.67(m,4H); 13 C NMR (100MHz, CDCl 3 ): δ202.7, 137.2, 136.6, 133.7, 132.4, 129.6, 128.8, 127.2, 125.5, 125.3, 124.3, 40.7, 40.3, 38.0, 34.0, 33.1, 28.6, 19.1; HRMS (ESI-TOF): theoretically calculated value: C 18 h 20 NaO[M+Na + ] 275.1406, measured value: 275.1406.
Embodiment 3
[0059] Embodiment 3: the preparation of compound 1-3
[0060]
[0061] The aryl iodide used was 2-methyliodobenzene (52.3 mg, 0.24 mmol), and other conditions were the same as in Example 1 to obtain compound I-3 (colorless oily liquid, yield 65%). 1 H NMR (400MHz, CDCl 3 ): δ9.50(t, J=3.0Hz, 1H), 7.04–7.01(m, 1H), 6.96–6.94(m, 2H), 3.15(dd, J=16.2, 3.2Hz, 1H), 2.85– 2.82(m,2H),2.53(dd,J=16.2,2.8Hz,1H),2.49(s,3H),2.04–1.94(m,1H),1.86–1.76(m,3H),1.50(s, 3H); 13 CNMR (100MHz, CDCl 3 ): δ203.4, 140.0, 138.0, 136.3, 131.2, 128.4, 126.3, 54.1, 41.7, 37.0, 32.3, 27.9, 23.8, 19.3; HRMS (ESI-TOF): Theoretical calculation value: C 14 h 18 NaO[M+Na + ] 225.1250, measured value: 225.1247.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com