Amphiphilic graft copolymer with catechol ligand as well as synthesis method and application thereof
A technology of grafting polymer and synthesis method, applied in the field of intelligent nanomicelle carrier, can solve problems such as toxic and side effects, drug leakage, destruction of micellar structure, etc., and achieve the effect of killing effect and stable existence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of mPEG-g-PCD-DA
[0053] Step 1: N-tert-butoxycarbonylethylenediamine (3.2g, 0.02mol) and N,N'-bis(acryloyl)cystamine (5.2g, 0.02mol) were dissolved in 20mL methanol / water (9 / 1 , v / v) in a mixed solvent. The reaction was protected from light and nitrogen, and reacted at 60° C. for 4 days. Subsequently, an excess of 10% N-tert-butoxycarbonylethylenediamine was added to continue the reaction for 1 day to consume unreacted carbon-carbon double bonds; after the reaction was completed, the solvent was removed under reduced pressure, and 16 mL of DCM / TFA (1 / 1, v / v ) mixed solution, stirred at room temperature for 2h to remove the protecting group. The reaction solution was added dropwise to excess diethyl ether, repeated 3 times. The solid obtained by filtration was dissolved in deionized water, dialyzed against deionized water for 24 hours (MWCO: 1000), and freeze-dried to obtain a white solid. The structural formula of the poly(N,N'-bis(acryloyl)cystamine-eth...
Embodiment 3
[0059] Synthesis of control polymer mPEG-g-PHD-DA
[0060] Except that 1,6-hexamethylenediamine is used as a raw material, other synthesis steps and reaction material ratios are the same as those of mPEG-g-PCD-DA.
[0061] The structural formula of the mPEG-g-PHD-DA is shown in formula (II);
[0062]
Embodiment 4
[0064] Assembly of CCLMs / SS micelles
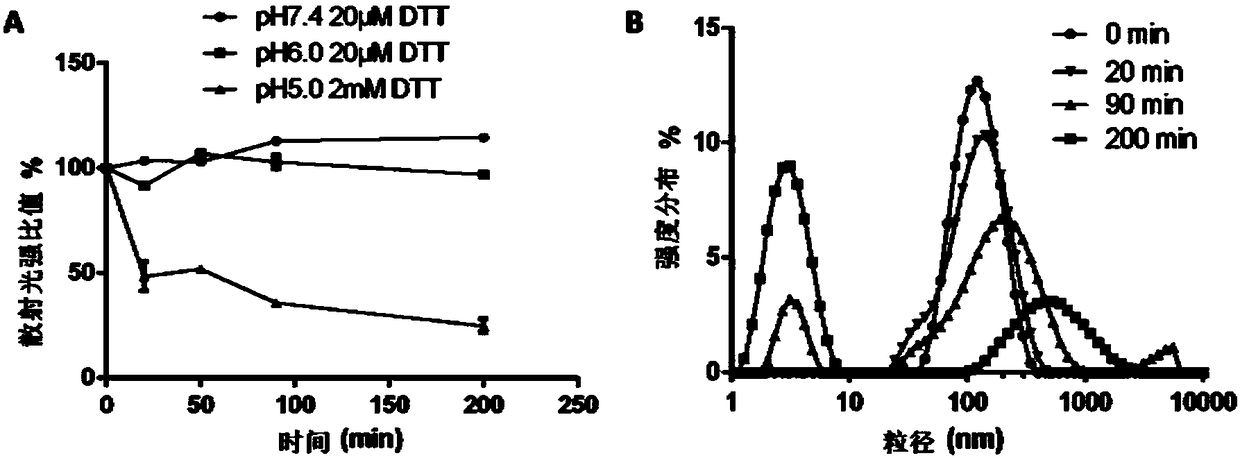
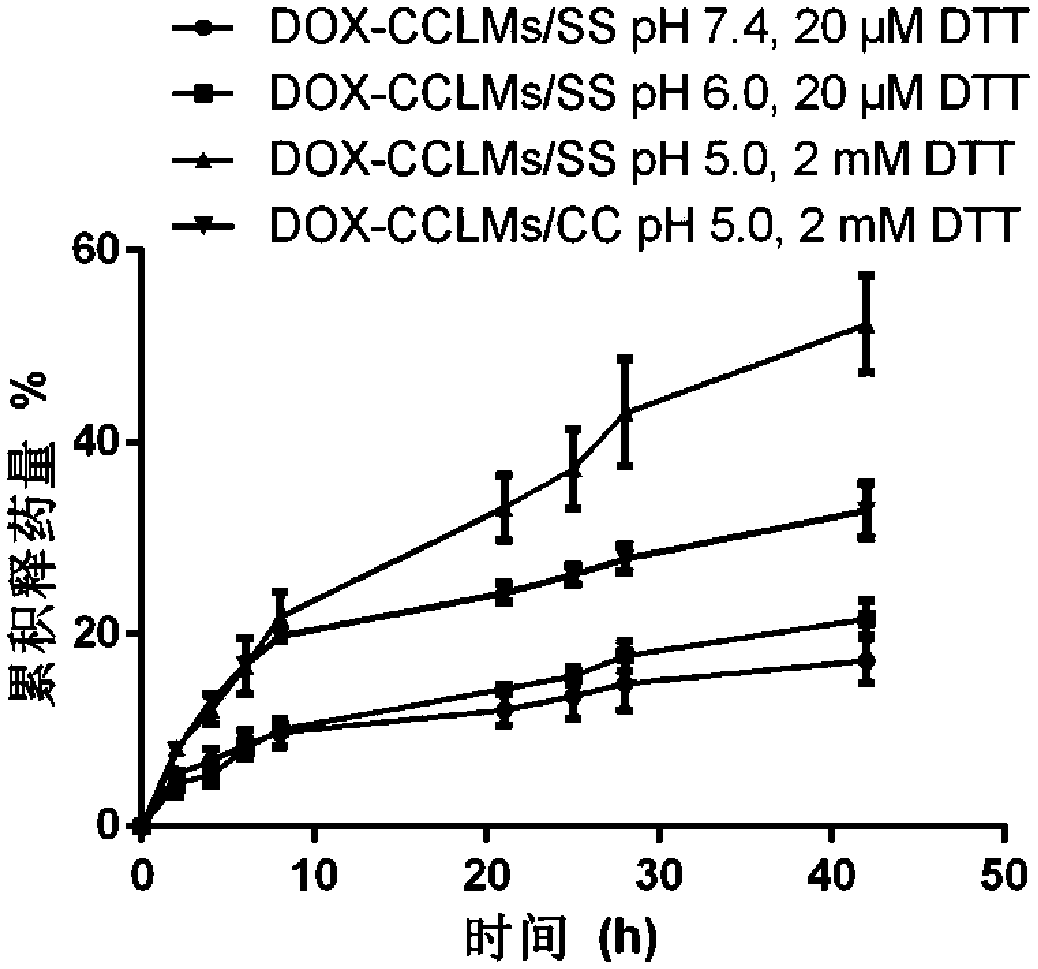
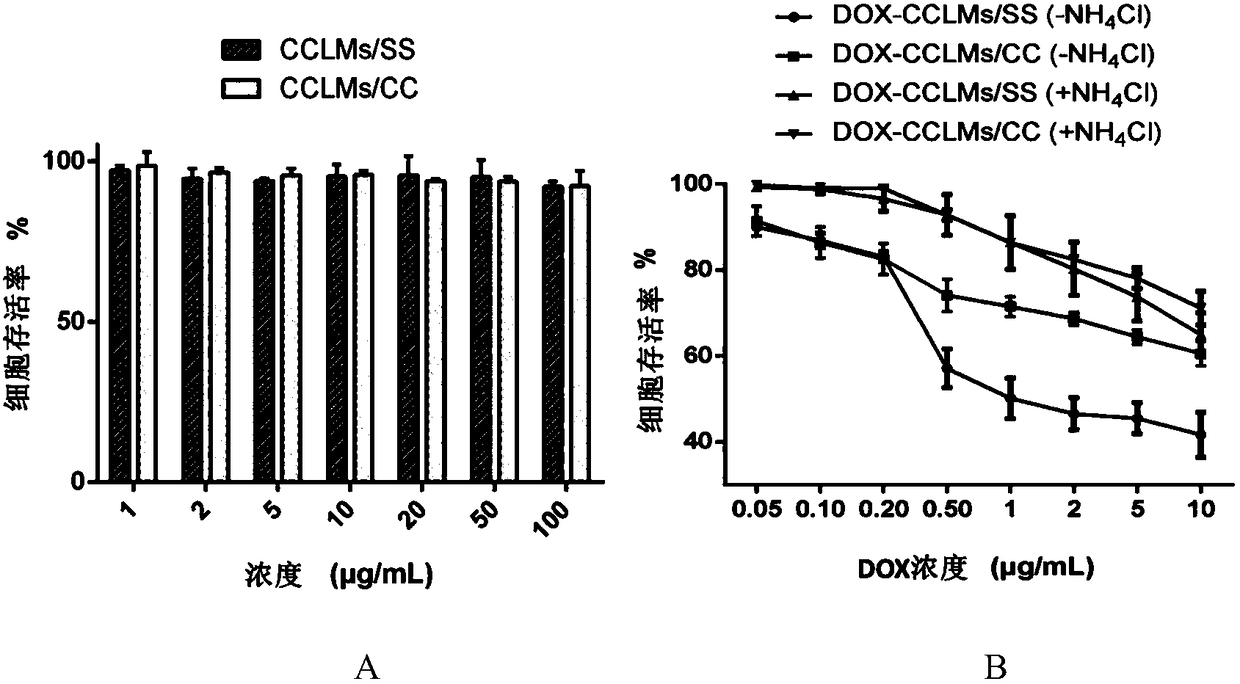
[0065] Weigh 20 mg of mPEG-g-PCD-DA solid and dissolve it in 2 mL of DMSO, and slowly inject it into 20 mL of deionized water with a micro-injection pump under stirring. It was then dialyzed against deionized water to remove DMSO. Under stirring, FeCl was added at a ratio of 2:1 molar ratio of catechol groups to iron ions. 3 solution (40mM). After stirring for 1 h, the pH was adjusted to 7.4 with 0.25M NaOH solution. Then deionized water was dialyzed to remove uncomplexed iron ions to obtain CCLMs / SS solution. like image 3 As shown in A, the average particle diameter measured by DLS is about 121 nm. like image 3 As shown in B, it is spherical in shape observed by TEM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com