Cinnamyl amino acid compound and application thereof
A technology of cinnamoyl amino acids and compounds, applied in the field of cinnamoyl amino acid compounds, can solve problems such as difficult to pass through the blood-brain barrier, concentration maintenance time period, and limited clinical application, and achieve clear pharmacological effects, small toxic and side effects, and inhibition of iNOS Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. In vitro anti-inflammatory activity of cinnamoyl amino acid compounds
[0025] 1.1 Experimental materials: BV-2 cells, DMEM cell culture medium, Gibico fetal bovine serum, 96-well cell culture plate, DMSO (Sigma), LPS (Sigma), NO detection kit (Beiyuntian), microplate reader.
[0026] 1.2 Experimental method:
[0027] BV-2 cells in 1 × 10 4 Cells / well were seeded in 96-well plates, and then grouped into groups; blank group, LPS model group, LPS+10 μM different test drug groups, and cultured for 24 hours. Then drugs and blank culture solution were added to the corresponding groups to continue culturing for 4 hours. Finally, except the blank group, each group was stimulated with LPS at a final concentration of 500 ng / mL for 24 hours. Finally, draw 50 μL of the supernatant to measure the NO content according to the requirements of the NO detection kit.
[0028] 1.3 Experimental results: such as figure 1 Shown, the amount of NO released. 4-Hydroxycinnamoyl-L-tyrosi...
Embodiment 2
[0031] 2. Cinnamoyl amino acid compounds inhibit iNOS in vitro
[0032] 2.1 Experimental materials: BV-2 cells, DMEM cell culture medium, Gibico fetal bovine serum, 96-well cell culture plate, DMSO (Sigma), LPS (Sigma), iNOS viability detection kit (Beiyuntian), microplate reader.
[0033] 2.2 Experimental method: BV-2 cells were mixed with 1×10 4 Cells / well were inoculated in 96-well plates, and then grouped into groups; blank group, LPS model group, LPS+(0.01-10 μM) different test drug groups, and cultured for 24 hours. Then drugs and blank culture solution were added to the corresponding groups to continue culturing for 4 hours. Finally, except the blank group, each group was stimulated with LPS at a final concentration of 500 ng / mL for 24 hours. Finally, iNOS activity was determined according to the requirements of the iNO detection kit.
[0034] 2.3 Experimental results:
[0035]
[0036] As shown in Table 1, the half inhibition IC of cinnamoyl amino acid compounds o...
Embodiment 3
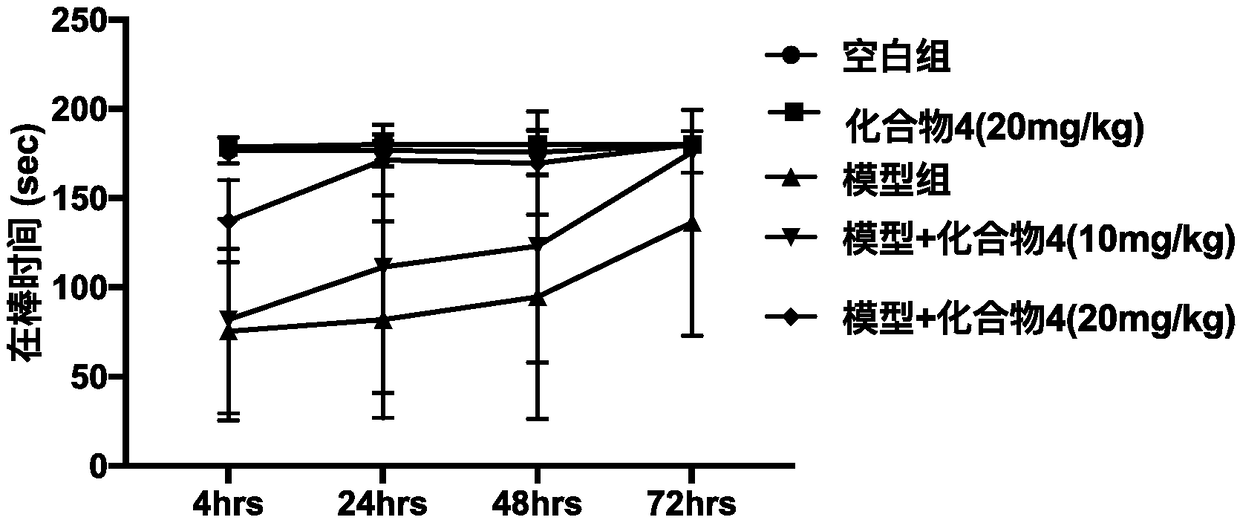
[0039] 3. Preferred compound 4-hydroxy-3-methoxycinnamoyl-L-tyrosine anti-Parkinson activity in vivo
[0040] 3.1 Experimental materials: 10-week-old C57BL / 6, rotary rod fatigue tester, MPTP (Sigma)
[0041] 3.2 Experimental grouping: Mice were randomly divided into 5 groups, 5 in each group: normal control group, diocortin alone group, model group, 4-hydroxy-3-methoxycinnamoyl-L-tyrosine Low-dose group (10 mg / kg), 4-hydroxy-3-methoxycinnamoyl-L-tyrosine high-dose group (20 mg / kg). Continuous pre-administration for 12 days, the blank group and the model group were given equal doses of PBS, and the model was made on the 13th day, and the model group and the drug group were given intraperitoneal injection of MPTP (20mg / kg) every 2h for a continuous injection of 4 hours. Second-rate. After modeling, conduct behavioral tests. The rotarod performance of mice was tested using the YLS-4C mouse rotarod instrument. 4, 24, 48, and 72 hours after MPTP modeling, the mice were placed o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com