A Xuesaitong pharmaceutical composition and its preparation method, preparation and application
A technology of Xuesaitong and its composition, which is applied in the field of Xuesaitong pharmaceutical composition and its preparation, can solve the problems of unfixed medicinal ingredients and composition, adverse reactions, unclearness, etc., and achieve reduction of myocardial infarction size and cardiac function. Impairment improvement, the effect of improving heart function indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0181] The preparation method of Xuesaitong pharmaceutical composition of the present invention, comprises the steps:
[0182] Step 1): Weigh notoginsenoside R1, place it in an ethanol solvent, stir and dissolve to obtain solution A;
[0183] Step 2): Weigh ginsenoside Rb2, ginsenoside Rb1, ginsenoside Rg1 and ginsenoside Rc, stir and dissolve in distilled water, heat to 50-70°C, add solution A while stirring, add activated carbon for needles, heat up and boil for 30 -50 minutes, let stand to cool to room temperature, filter out activated carbon;
[0184] Step 3): Put the above filtrate in a vacuum freeze dryer, cool down to -40~-30°C, pre-freeze for 3~5 hours, then control the temperature at -25~-20°C, pressure 10~20Pa, sublimation and dry for 3~ 5 hours, and then control the temperature at 30-50°C, pressure at 1-10Pa, and analyze and dry for 4-6 hours to obtain it.
[0185] In step 1), the mass ratio of notoginseng saponin R1 to ethanol solvent is 0.05-5:100, and the volum...
Embodiment 11
[0251]The preparation of embodiment 1 1# pharmaceutical composition
[0252] Raw material composition:
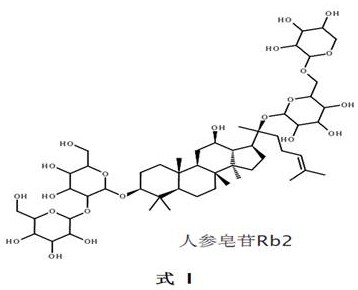
[0253] Ginsenoside Rb2 54.6 g
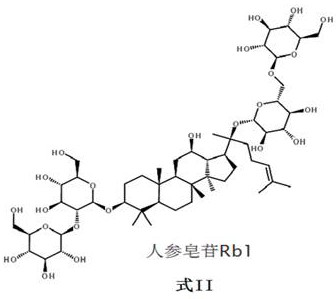
[0254] Ginsenoside Rb1 21.1 g
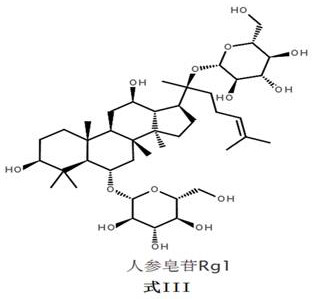
[0255] Ginsenoside Rg1 11.3 g
[0256] Ginsenoside Rc 8.5 g
[0257] Notoginsenoside R1 4.5g
[0258] Total 100g
[0259] Preparation:
[0260] 1) Weigh 4.5 g of notoginseng saponin R1, place it in 100 g of ethanol solvent with a concentration of 50% by volume, stir and dissolve to obtain solution A;
[0261] 2) Weigh 54.6 g of ginsenoside Rb2, 21.1 g of ginsenoside Rb1, 11.3 g of ginsenoside Rg1 and 8.5 g of ginsenoside Rc, stir and dissolve in 900 g of distilled water, heat to 50-70°C, add solution A while stirring, Add 2.0g of activated carbon for needles, heat up and boil for 30-50 minutes, let it stand to cool to room temperature, filter out the activated carbon;
[0262] 3) Put the above filtrate in a vacuum freeze dryer, cool down to -40~-30℃ at a cooling rate of 5℃ / h, pre-freeze for 3~5 hours, ...
Embodiment 2
[0263] The preparation of embodiment 2 2# pharmaceutical composition
[0264] Raw material composition:
[0265] Ginsenoside Rb2 20.5 g
[0266] Ginsenoside Rb1 47.8 g
[0267] Ginsenoside Rg1 23.4 g
[0268] Ginsenoside Rc 6.7 g
[0269] Notoginsenoside R1 1.6 g
[0270] Total 100g
[0271] Preparation:
[0272] 1) Weigh 1.6 g of notoginseng saponin R1, place it in 100 g of ethanol solvent with a concentration of 80% by volume, stir and dissolve to obtain solution A;
[0273] 2) Weigh 20.5 g of ginsenoside Rb2, 47.8 g of ginsenoside Rb1, 23.4 g of ginsenoside Rg1 and 6.7 g of ginsenoside Rc, stir and dissolve in 950 g of distilled water, heat to 50-70°C, add solution A while stirring, Add 1.0g of activated carbon for needles, heat up and boil for 30-50 minutes, let it stand and cool to room temperature, and filter out the activated carbon;
[0274] 3) Put the above filtrate in a vacuum freeze dryer, cool down to -40~-30℃ at a cooling rate of 15℃ / h, pre-freeze for 3~...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com