Clean production method of medicine ponatinib intermediate for treating leukemia
A technology of precursor and modified water, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino compounds, etc., can solve the problems of low product yield and large environmental pollution, and achieve high product selectivity and enhanced conversion. rate, mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
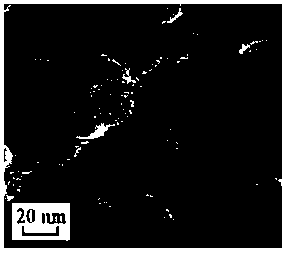
Image
Examples
Embodiment 1
[0042] Preparation of cerium-modified hectorite supported Pd catalyst:
[0043] 1) Preparation of cerium-modified hectorite material:
[0044] 1-1) Add 90mmol ethyl orthosilicate to 200ml ethanol, stir at room temperature for 15min; add dropwise 100ml MgCl with a concentration of 0.5mol / L 2 .6H 2 0 aqueous solution, stir at room temperature for 1-2 hours after the dropwise addition to form a uniform silicon-magnesium mixture; add 1mol / L ammonia solution to the silicon-magnesium mixture dropwise, adjust the pH to 11, and stir at 70°C for 1-2 days Si-Mg precipitates are formed;
[0045] 1-2) Cool down to room temperature, centrifuge at 1000rpm to obtain particles, disperse the particles into 300ml purified water, then add 10mmol LiOH and 5mmol Ce(NO 3 ) 3 .6H 2 O Stir evenly, add 1mol / L ammonia solution to adjust the pH to 11.0, stir evenly to obtain a solid dispersion, and then transfer the solid dispersion to a hydrothermal reaction kettle at 130-140 After hydrothermal r...
Embodiment 1-A
[0051] The hectorite-supported Pd catalyst was prepared by a single factor method, that is, no Ce(NO 3 ) 3 .6H 2 O, the rest are exactly the same as in Example 1, and the prepared catalyst is abbreviated as Pd / H.
Embodiment 2
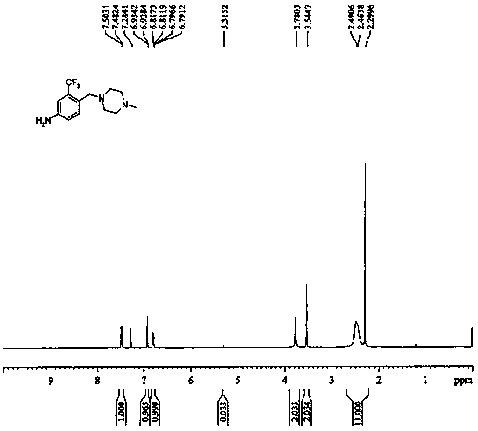
[0053] The cerium-modified hectorite loaded Pd catalyst (abbreviated as Pd / Ce-H) prepared in Example 1, the cerium-modified hectorite material (abbreviated as Ce-H) and the Pd prepared in Example 1-A thereof / H and commercially available 10.0%wt Pd / C as catalysts (abbreviated as Pd / C), catalyzed 4-(4-methylpiperazine-1-methylene)-3-trifluoromethyl-1-nitro Benzene prepares 3-trifluoromethyl-4-[(4-methylpiperazin-1-yl) methyl] aniline, and the reaction conditions are as follows:
[0054] Catalytic hydrogen transfer: add substrate 4-(4-methylpiperazine-1-methylene)-3-trifluoromethyl-1-nitrobenzene (10mmol, 3.03g), 40ml of ethanol, formic acid into the reactor Ammonium (80mmol, 5.04g), catalyzer (181mg, 6.0%wt), be warming up to 60-70 ℃ of reaction, HPLC detects each catalyst reaction system reaction liquid situation, treats that when reaction reaches equilibrium, counts each catalytic system substrate 4-(4 - Conversion rate of methylpiperazine-1-methylene)-3-trifluoromethyl-1-ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com