Magnoflorine preparations and preparation methods and application
A technology of magnolia alkaloid and magnolia alkaloid phospholipid, applied in the fields of health care products and medicine, can solve the problems of low bioavailability and low fat solubility of magnolia alkaloid, achieve high bioavailability, improve oral availability and take effect quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation method of Magnolin
[0041] (1) Preparation of crude extract:
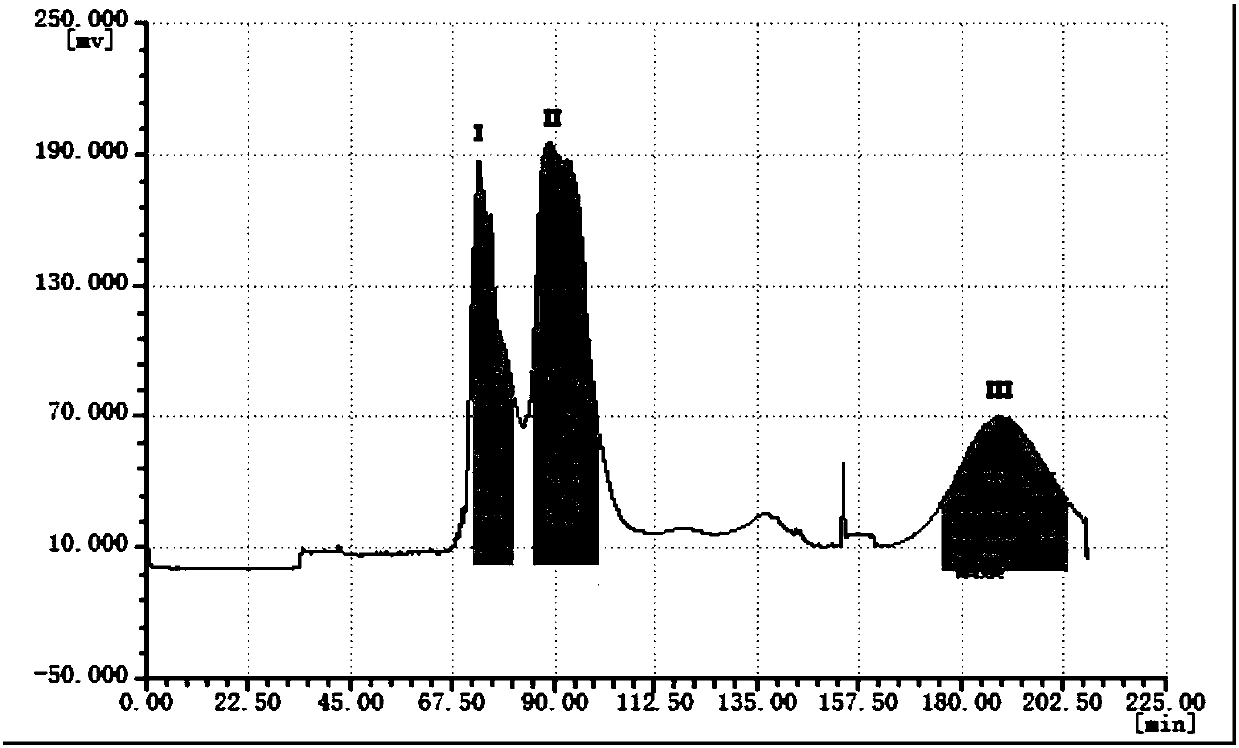
[0042] Grind 250g jujube kernels, pass through a 40-mesh sieve, degrease with petroleum ether, add 1200ml, 800ml, 600ml of 70% ethanol aqueous solution, heat and reflux for extraction 3 times, each time for 1 hour, filter, and combine the extracts , the filtrate is concentrated with ethanol until it has no alcohol smell, then dissolved with 2 times the amount of water, extracted 5 times with n-butanol, the extract is concentrated under reduced pressure, and the solvent is recovered to obtain the n-butanol crude extract, which is the sample to be separated. figure 1 shown.
[0043](2) Adopt high-speed countercurrent chromatography to separate magnolialine and spinosin, 6 "'-feruloylspinosin:
[0044] Take n-butanol, ethyl acetate and water at a volume ratio of 2:3:5, place them in a separatory funnel to prepare a two-phase solvent system, take the upper phase as the stationary phase, and the lo...
Embodiment 2
[0049] Pharmacodynamic experiment of magnolanine on CUMS depression and insomnia mouse model
[0050] 1 Experimental materials
[0051] 1.1 Experimental animals
[0052] Healthy male ICR mice (weight 20±2g) were purchased from the Experimental Animal Center of the Institute of Radiological Medicine, Chinese Academy of Medical Sciences; they were bred for 3 days to adapt to the environment before the experiment. The room temperature is 22±2°C, and the relative humidity is 65% to 70%. Sunlight for 12 hours, free diet and water intake.
[0053] 1.2 Reagents and medicines:
[0054] Magnolin, spinosin (self-made in this laboratory); venlafaxine hydrochloride sustained-release capsules (Chengdu Kanghong Pharmaceutical Group Co., Ltd.)
[0055] 1.3 Instruments
[0056] Stopwatch (Switzerland Hoyer-Olidas factory);
[0057] AUX 120 analytical balance (Shimadzu (Hong Kong) Co., Ltd.);
[0058] ZH-YLS-1A animal autonomous activity recorder (Anhui Zhenghua Biological Instrument Eq...
Embodiment 3
[0108] Preparation and detection of magnolia alkali phospholipid complex
[0109] 1 Experimental materials
[0110] 1.1 Drugs and reagents
[0111] Magnolin (made in laboratory, purity>98%);
[0112] Soy lecithin (Shanghai Lanji Biological Co., Ltd., batch number: 20160628);
[0113] Absolute ethanol, dichloromethane, methanol, ethyl acetate, n-butanol (analytical grade, Tianjin Benchmark Chemical Reagent Co., Ltd.);
[0114] 1.2 Instruments
[0115] RCT basic magnetic stirrer (Guangzhou Yike Laboratory Technology Co., Ltd.);
[0116] 214-type differential scanning calorimeter (Germany Netzsch);
[0117] Model 380 Fourier transform infrared spectrometer (Hitachi High-Tech Corporation, Japan);
[0118] HT7700 Transmission Electron Microscope (Hitachi High-Tech Corporation, Japan);
[0119] Agilent1100 high performance liquid chromatography (Agilent Technologies Co., Ltd.);
[0120] AUX120 analytical balance (Shimadzu (Hong Kong) Co., Ltd.);
[0121] 2 Experimental meth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Average hydrated particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com