Camellia oleifera active glycoprotein with modified configuration, and preparation method and application of same
A glycoprotein and structural modification technology, which is applied in the preparation method of peptides, medical preparations containing active ingredients, and peptide/protein components, etc., can solve the structure-activity relationship between the structure and functional activity of Camellia oleifera glycoproteins. The refined quantitative utilization of proteins lacks effective and extensive data support, mechanism research gaps, etc., to achieve the effect of efficient optimization of structure-effectiveness modification methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
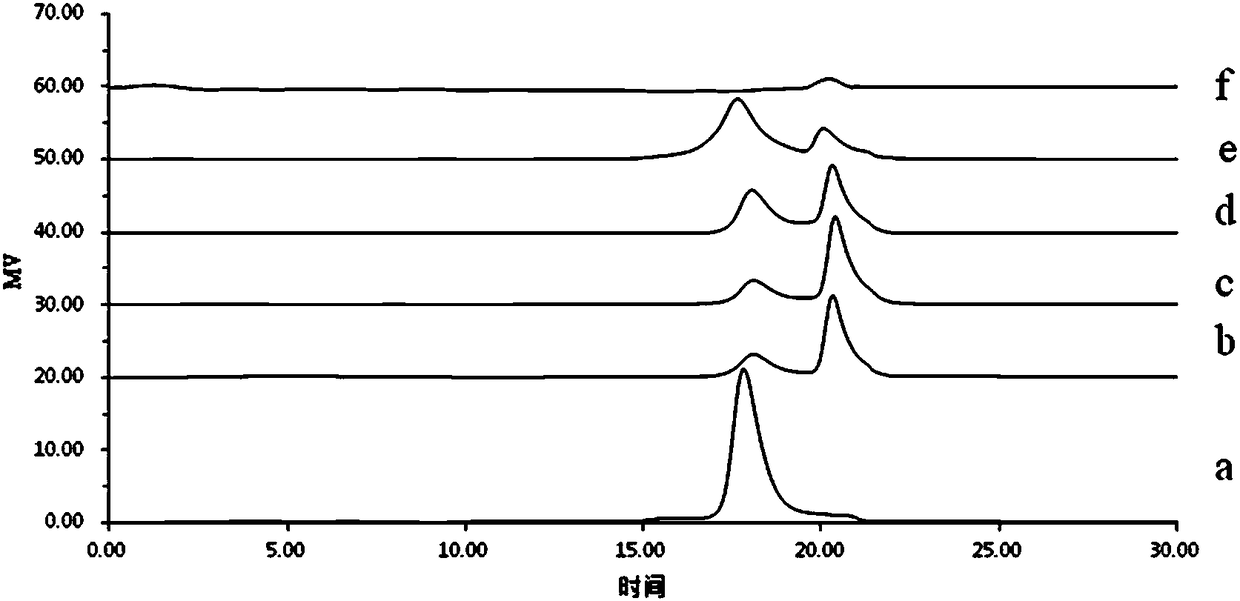
Image
Examples
Embodiment 1
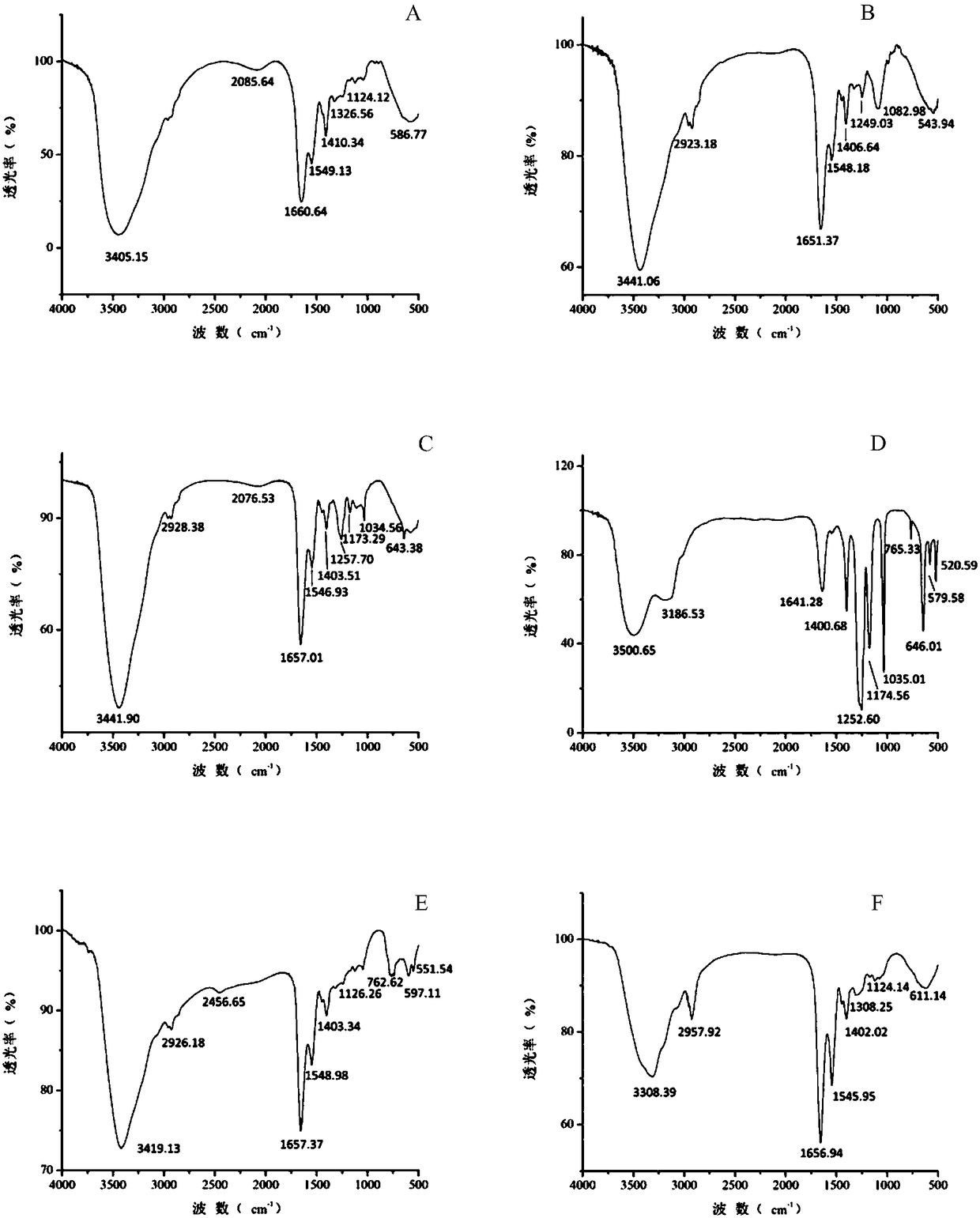
[0023] Modification of camellia oleifera glycoprotein by trifluoromethanesulfonic acid (TFMS) method
[0024] Camellia oleifera is pulverized and degreased with petroleum ether, and the saponin is removed with 90% ethanol by volume fraction, and dried for later use. According to the liquid-to-solid ratio of 20mL / g, extract with water at 60°C for 3 hours, then filter, add absolute ethanol to the filtrate to make the final volume concentration of ethanol 70%, and freeze-dry the precipitate after centrifugation. The dried product was dissolved in 0.01mol / L Tris-HCl buffer (pH 8, containing 0.02mol / LNaCl) and separated and purified with DEAE Sepharose F.F. ion exchange column. Part of the 0.2mol / L NaCl eluate, after the eluate was dialyzed to remove salt, was separated and purified by Sephadex G-100 gel filtration column, the first peak was collected, and then passed through the AKTA protein separation and purification instrument Superdex TM The G-75 gel column was used for sepa...
Embodiment 2
[0027] Periodic acid (NaIO 4 ) to transform camellia oleifera glycoprotein
[0028] Camellia oleifera is pulverized and degreased with petroleum ether, and the saponin is removed with 90% ethanol by volume fraction, and dried for later use. According to the ratio of liquid to material of 20mL / g, extract with water at 70°C for 2.5h and then filter, add absolute ethanol to the filtrate to make the final volume concentration of ethanol 80%, centrifuge and freeze-dry the precipitate. The dry matter was dissolved in 0.02mol / L Tris-HCl buffer solution (pH 8, containing 0.02mol / L NaCl) and separated and purified with DEAE Sepharose F.F. ion exchange column, based on the results of in vitro anti-tumor and antioxidant activities. Collect the part of the 0.3mol / L NaCl eluate. After the eluate is dialyzed to desalt, it is separated and purified by Sephadex G-100 gel filtration column, the first peak is collected, and then passed through the AKTA protein separation and purification instr...
Embodiment 3
[0031] Enzymatic transformation of camellia oleifera glycoprotein with PNGase F
[0032] Camellia oleifera is pulverized and degreased with petroleum ether, and the saponin is removed with 90% ethanol by volume fraction, and dried for later use. According to the liquid-to-solid ratio of 20mL / g, extract with water at 80°C for 2 hours, then filter, add absolute ethanol to the filtrate to make the final volume concentration of ethanol 85%, and freeze-dry the precipitate after centrifugation. The dried product was dissolved in 0.03mol / L Tris-HCl buffer (pH 8, containing 0.02mol / LNaCl) and separated and purified with DEAE Sepharose F.F. ion exchange column. Part of the 0.4mol / L NaCl eluate, after the eluate was dialyzed to remove salt, was separated and purified by Sephadex G-100 gel filtration column, the first peak was collected, and then passed through the AKTA protein separation and purification instrument Superdex TM The G-75 gel column was used for separation and purificati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com