Camellia oleifera active glycoprotein with structure-effect modification and its preparation method and application
A technology for glycoprotein and structural transformation, which is applied in the preparation method of peptides, medical preparations containing active ingredients, peptide/protein components, etc. It can solve the structure-activity relationship between the structure and functional activity of camellia oleifera glycoproteins has not yet been clarified, and the structure-activity relationship of camellia oleifera sugar Protein fine quantification takes advantage of problems such as lack of effective and extensive data support, blank mechanism research, etc., to achieve the effect of efficient optimization of structure-efficiency modification methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
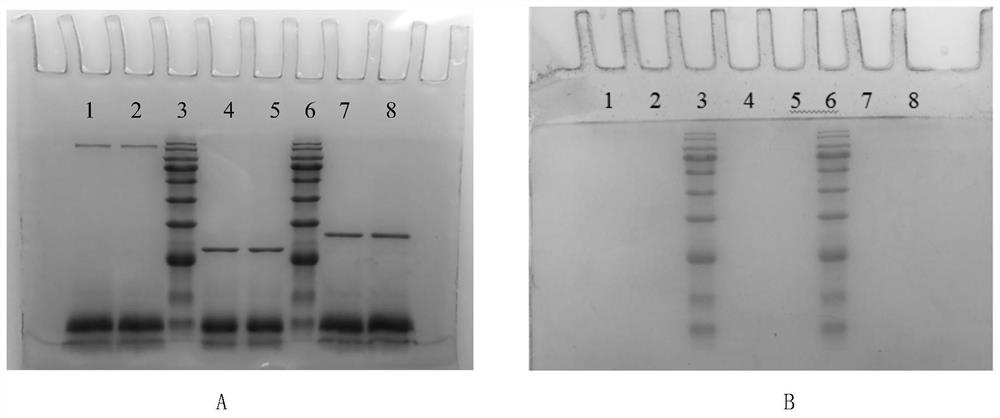
[0023] Modification of camellia oleifera glycoprotein by trifluoromethanesulfonic acid (TFMS) method
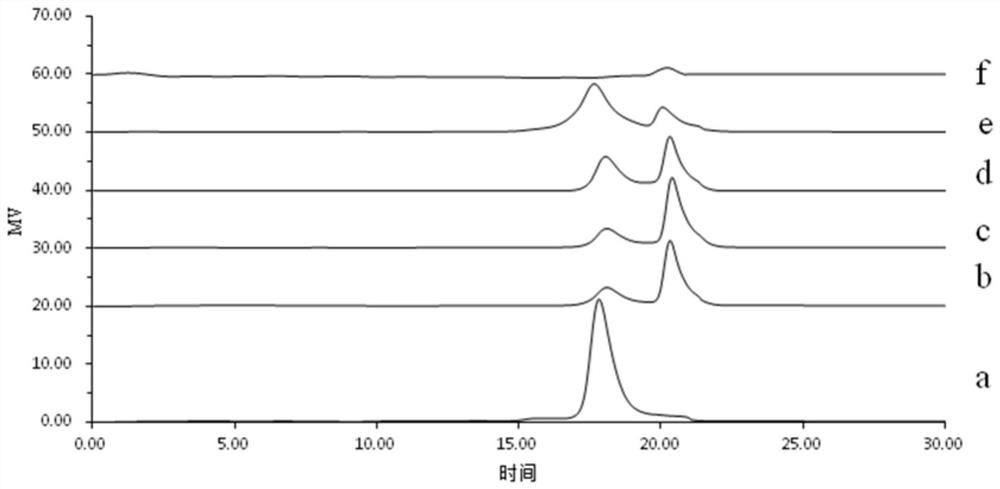
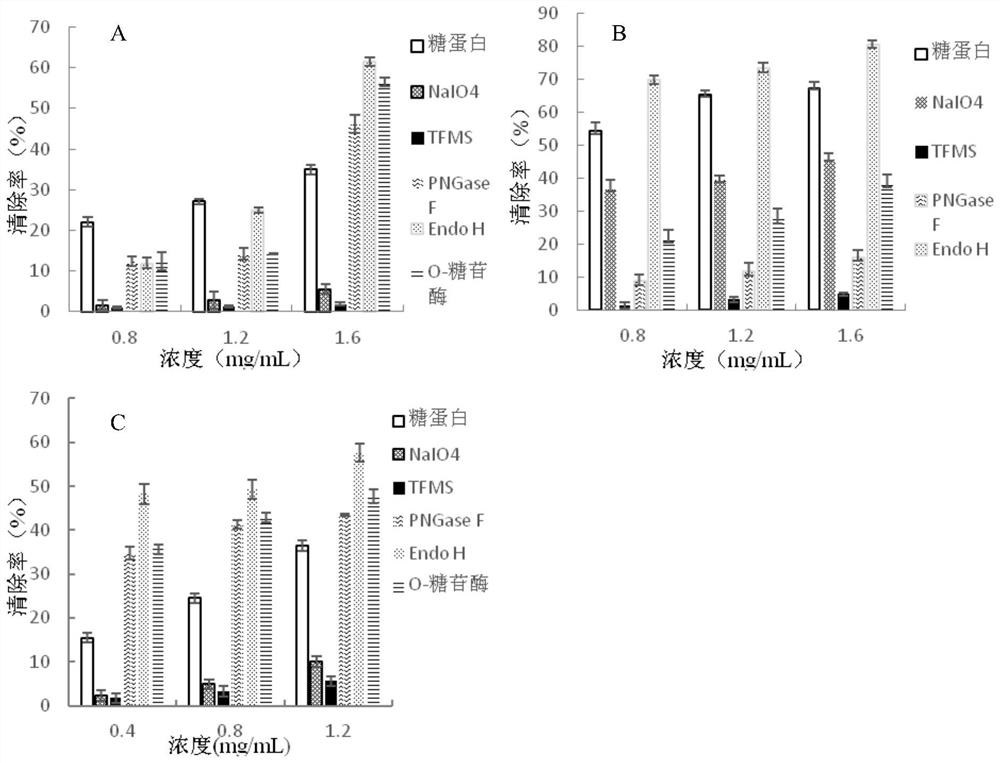
[0024] Camellia oleifera is pulverized and degreased with petroleum ether, and the saponin is removed with 90% ethanol by volume fraction, and dried for later use. According to the liquid-to-solid ratio of 20 mL / g, extract with water at 60°C for 3 hours, then filter, add absolute ethanol to the filtrate to make the final volume concentration of ethanol 70%, and freeze-dry the precipitate after centrifugation. The dried product was dissolved in 0.01mol / L Tris-HCl buffer (pH 8, containing 0.02mol / LNaCl) and separated and purified with DEAE Sepharose F.F. ion exchange column. Part of the 0.2mol / L NaCl eluate, after the eluate was dialyzed to remove salt, was separated and purified by SephadexG-100 gel filtration column, the first peak was collected, and then passed through the AKTA protein separation and purification instrument Superdex TMThe G-75 gel column was used for separa...
Embodiment 2
[0027] Periodic acid (NaIO 4 ) to transform camellia oleifera glycoprotein
[0028] Camellia oleifera is pulverized and degreased with petroleum ether, and the saponin is removed with 90% ethanol by volume fraction, and dried for later use. According to the liquid-to-solid ratio of 20 mL / g, extract with water at 70°C for 2.5 hours, then filter, add absolute ethanol to the filtrate to make the final volume concentration of ethanol 80%, and freeze-dry the precipitate after centrifugation. The dried product was dissolved in 0.02mol / L Tris-HCl buffer (pH 8, containing 0.02mol / L NaCl) and separated and purified with DEAE Sepharose F.F. ion exchange column, based on the results of in vitro anti-tumor and antioxidant activities. Collect the part of the 0.3mol / L NaCl eluate. After the eluate is dialyzed to desalt, it is separated and purified by Sephadex G-100 gel filtration column, the first peak is collected, and then passed through the AKTA protein separation and purification inst...
Embodiment 3
[0031] Enzymatic transformation of camellia oleifera glycoprotein with PNGase F
[0032] Camellia oleifera is pulverized and degreased with petroleum ether, and the saponin is removed with 90% ethanol by volume fraction, and dried for later use. According to the liquid-to-solid ratio of 20 mL / g, add water to extract at 80°C for 2 hours, then filter, add absolute ethanol to the filtrate to make the final volume concentration of ethanol 85%, and freeze-dry the precipitate after centrifugation. The dried product was dissolved in 0.03mol / L Tris-HCl buffer (pH 8, containing 0.02mol / LNaCl) and separated and purified with DEAE Sepharose F.F. ion exchange column. Part of the 0.4mol / L NaCl eluate, after the eluate was dialyzed to remove salt, was separated and purified by SephadexG-100 gel filtration column, the first peak was collected, and then passed through the AKTA protein separation and purification instrument Superdex TM The G-75 gel column was used for separation and purifica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com