Optimized polypeptide for a subunit vaccine against avian reovirus
An avian reovirus and vaccine technology, which is used in viral peptides, antiviral agents, veterinary vaccines, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1. SC protein expression
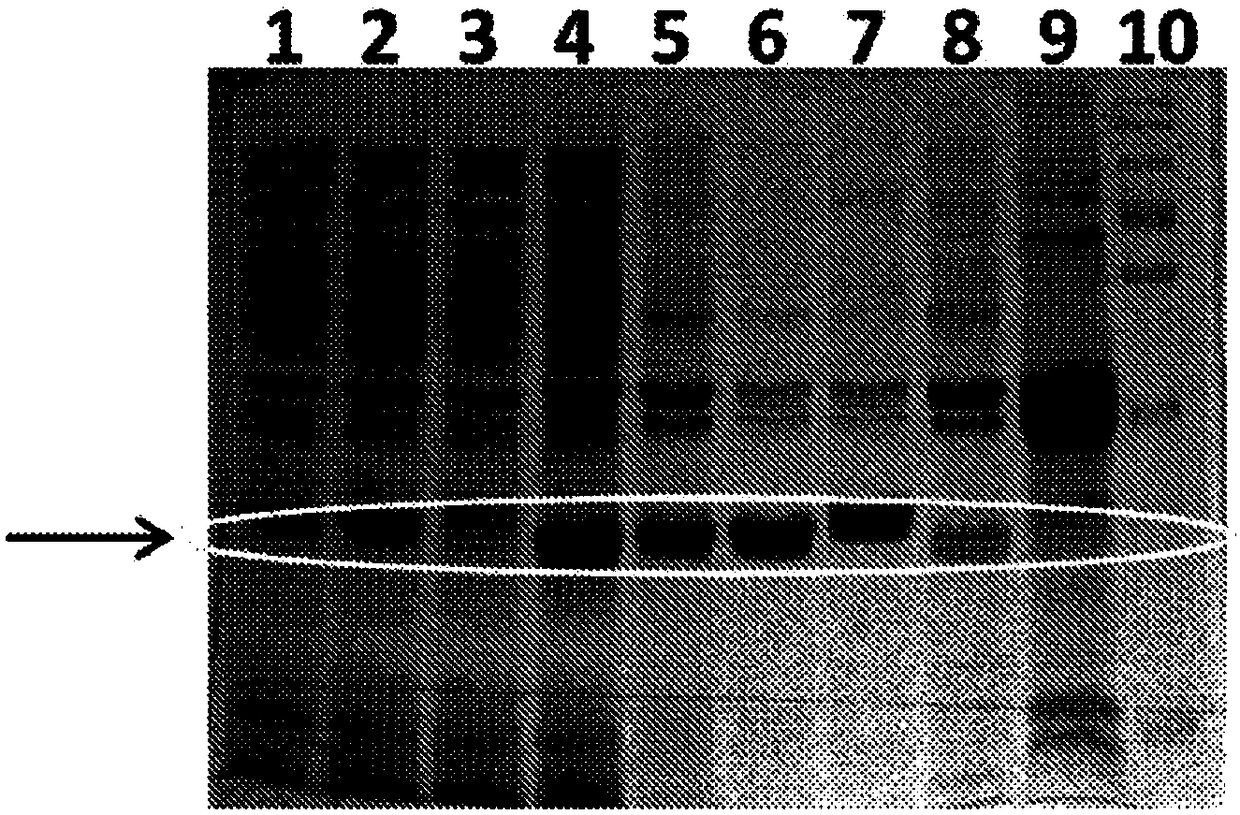
[0097] After analyzing the SC protein structure, a cDNA fragment encoding three polypeptide residues: 1-326, 122-326, 192-326 was generated, cloned and expressed in E. coli. A polyhistidine tag was added at the 5' end to allow detection and purification of the expressed protein. The resulting protein was partially purified. As determined on a 12% SDS-polyacrylamide gel, the expressed SC fragments were 36kD, 23kD and 15kD with the expected sizes, respectively.
[0098] Figure 1 shows the efficient expression of the 122-326 fragment from four different reovirus isolates: SC1133, SC528, SC5223 and SC5215.
Embodiment 2
[0099] Example 2. Antibody Response to Vaccination
[0100] After vaccination, the immunogenicity of the SC protein and the ability of the antibodies generated to detect the virus were tested by ELISA. Using SC122-326 as the antigen, antibodies obtained after vaccination with the proteins SC122-326 and SC192-326 detected the antigen at a high titer of 1:800,000. The average value of bird titers in this group was significantly higher than that of the negative control group (P figure 2 ).
[0101] Antibody levels against the whole virus reached 0.2 or less after inoculation with SC192-326 or the negative control, whereas antibodies obtained after inoculation with SC1-326 or SC122-326 were positive (0.25 and 0.5, respectively) ( image 3 ). The S / P of SC122-326 group was significantly higher than other groups.
[0102] Similarly, following vaccination, SC122-326 fragments of four different RI groups (SEQ ID NO: 2, 3, 4 and 5) were tested for immunogenicity and the ability of t...
Embodiment 3
[0103] Example 3. Cell Proliferation
[0104] Splenocytes from vaccinated chickens were examined for antigen-specific cell proliferation. Cells were treated with ConA as a control for T cell proliferation, LPS as a control for T cell proliferation (positive control), PBS (negative control) and ARV (specific antigen). In the CTB assay, the measured absorbance is proportional to the number of viable cells. Results are expressed as SI (mitogen or antigen stimulated / non-stimulated cells).
[0105] Positive control treatments exhibited proliferation after stimulation. Cells stimulated with ARV showed significant differences among treatment groups. Compared with the negative control, the cells in the group immunized with virus or SC122-326 showed significant proliferation (P Figure 4 ).
[0106] Similarly, antigen-specific cell proliferation was examined in splenocytes from chickens vaccinated with combinations comprising polypeptides of SEQ ID NO: 2, 3, 4 and 5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com