Multi-epitope combined peptide used for treating and preventing human papillomavirus infection and related diseases
A multi-epitope and epitope peptide technology, applied in the direction of viral peptides, viruses, hybrid peptides, etc., can solve the problems of unsatisfactory effect, poor effect, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Multi-epitope combination peptide induces and activates HPV-specific DC-CIK immunity
[0041] Experimental group: the multi-epitope combination peptide fragment HSP70 407-424-AAA-HPV16 E7 11-19 artificially synthesized by Fmoc solid-phase synthesis method. The HSP70407-424-AAA-HPV16 E7 11-19 was dissolved in PBS at a concentration of 1 mg / ml, filtered and used for later use.
[0042] Control group: HPV 16E7 11-19 epitope peptide of HPV16E7 T cells artificially synthesized by Fmoc solid-phase synthesis method. The HPV16 E7 11-19 was dissolved in PBS to a concentration of 1 mg / ml, filtered and used for later use.
[0043] The Ficoll density gradient centrifugation method separates peripheral blood mononuclear cells, the purity is above 90%, the harvest rate can reach 80-90%, and the percentage of living cells is above 95%.
[0044] (1) Preparation of DC-CIK cells
[0045] After the PBMCs were washed twice with 0.9% normal saline, the cell concentration was a...
Embodiment 2
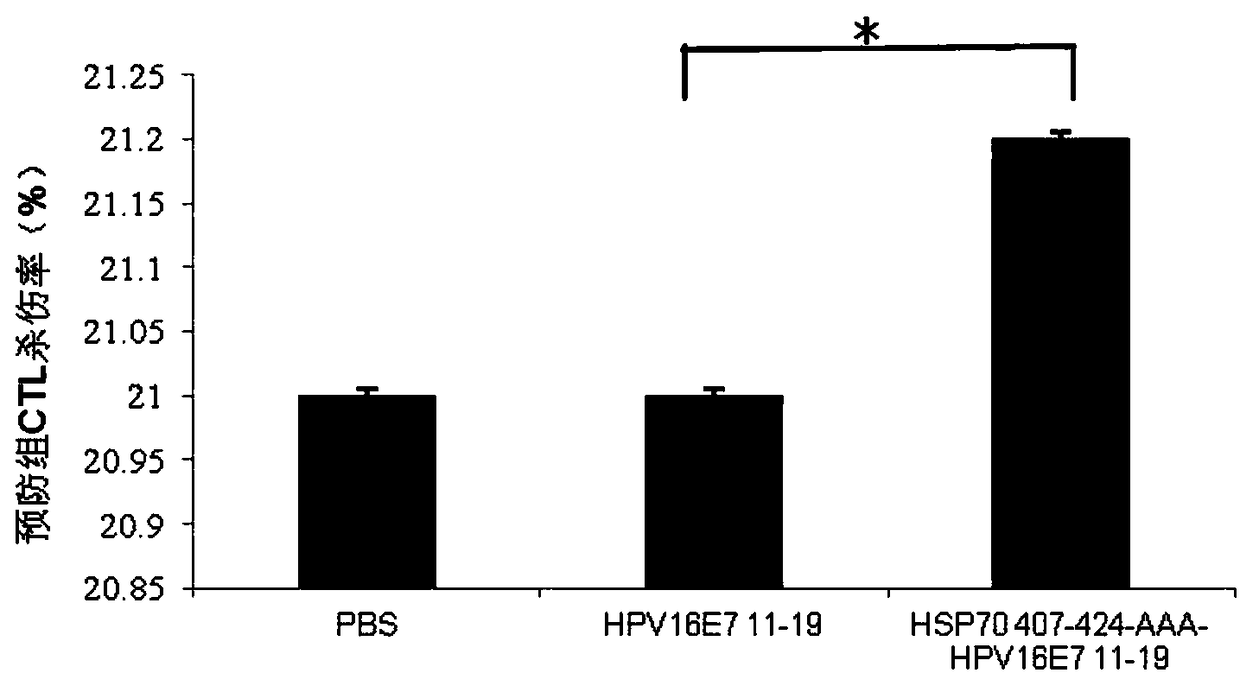
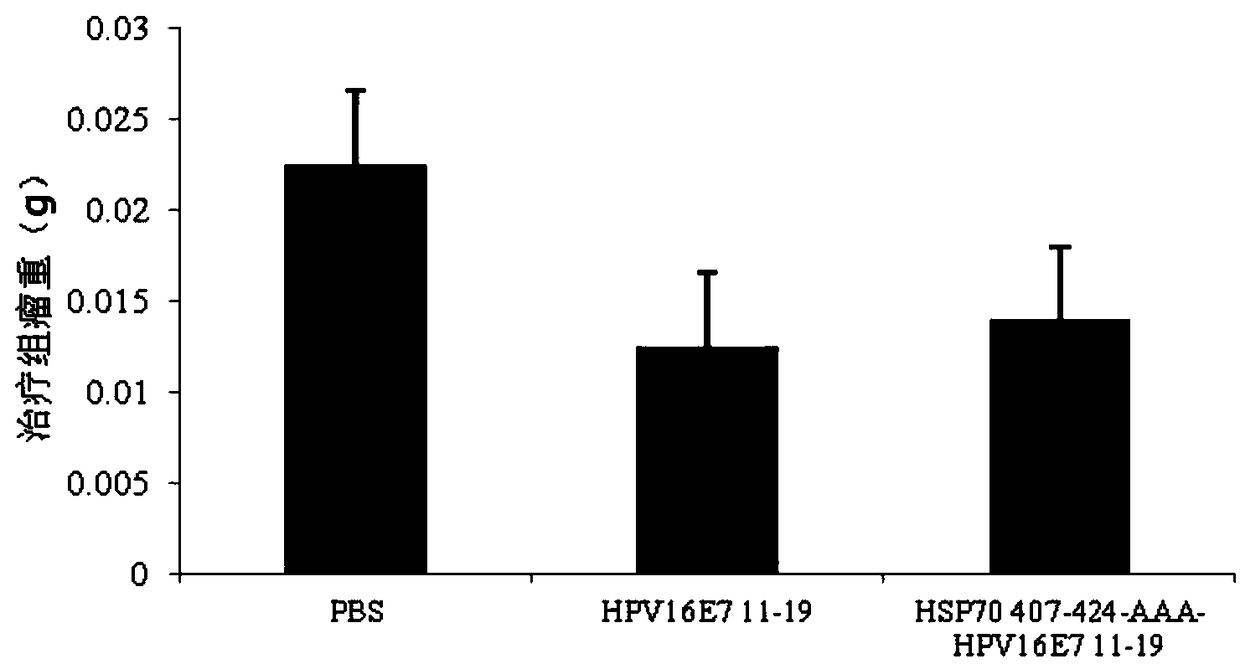
[0075] Embodiment 2 Mouse tumor-bearing model animal test
[0076] The 6-8 week-old, female, C57BL / 6 mice used in the present invention were randomly divided into 3 groups, namely blank group, control group and test group. There were 35 mice in each group (15 mice were used for the prevention model, 15 mice were used for the treatment model, and the other 5 mice were used for the re-challenge test of transplanted tumors), of which 15 mice were used for the prevention model or the treatment model. Of the mice, 5 were tested for tumor morphology, 5 for survival rate testing, and 5 for immunological testing. Specifically, the immune substance injected in the test group was HSP70 407-424-AAA-HPV16E7 11-19 multi-epitope combination peptide vaccine; the immune substance injected in the control group was HPV16E7 11-19 T cell antigen epitope peptide vaccine; the blank group was injected with Aseptic PBS was used as a blank control for the prevention and treatment of HPV16-infected mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com