Method for synthesizing bendamustine hydrochloride intermediate
A technology of bendamustine hydrochloride and a synthesis method, which is applied in the field of chemical synthesis, can solve the problems of low selectivity of reduction, a large amount of sodium sulfide waste water, environmental pollution and the like, and achieves the advantages of shortening production cycle, short route and reducing cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
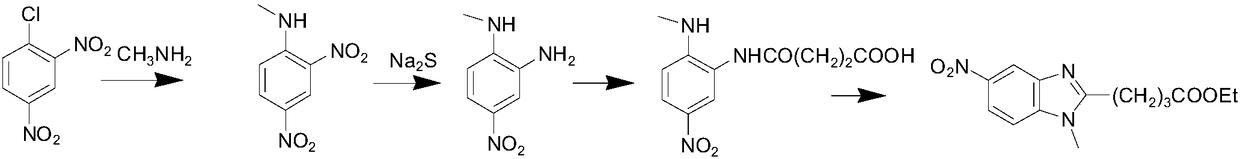
Method used
Image
Examples
Embodiment 1
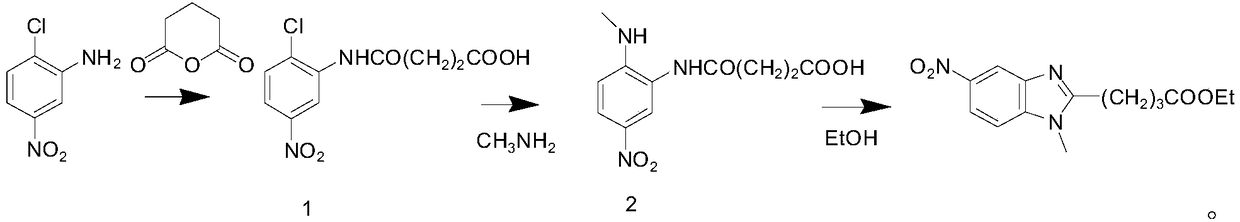
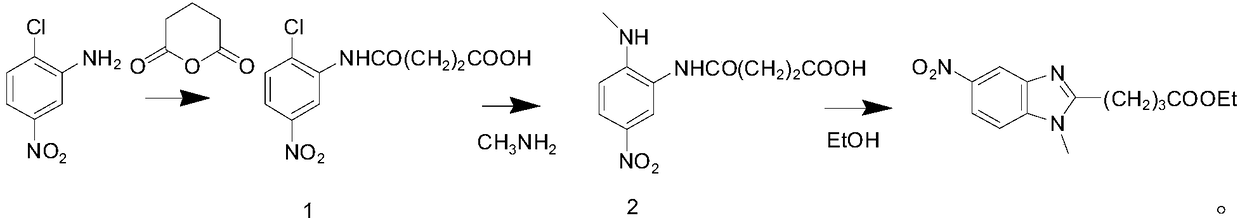
[0018] Take 51.6g of 2-chloro-5-nitroaniline into a three-neck flask, add 500ml of toluene, add 36g of glutaric anhydride at room temperature, heat to 80°C for 3 hours, cool to room temperature, filter, and filter cake at 70°C After drying, 145 g of a yellow solid intermediate was obtained, with a yield of 95%.
[0019] Add intermediate 1 obtained above into 150ml of 40% methylamine aqueous solution, heat to 50°C for 3 hours, cool to room temperature, adjust pH to 4-5 with 2N hydrochloric acid, precipitate a yellow solid, stir for 1 hour, filter, filter The cake was rinsed with toluene and dried at 70°C to obtain 40.5 g of yellow solid intermediate 2 with a yield of 91.7%.
[0020] Put the intermediate 2 prepared above into a three-necked flask, add 365ml of absolute ethanol, add 12ml of 98% concentrated sulfuric acid dropwise under stirring, heat to 80°C and reflux for 3h after the dropwise addition, cool to 45-50°C, pour the reaction solution into In saturated aqueous potas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com