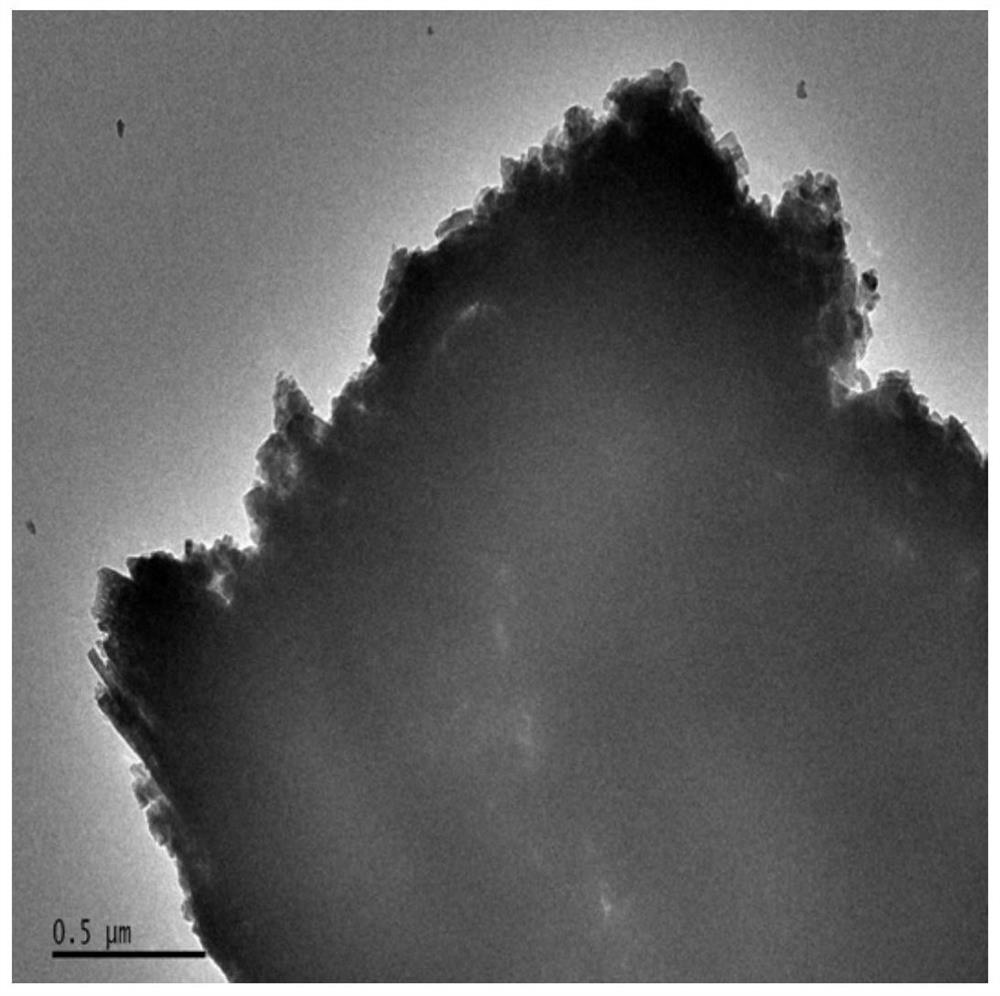
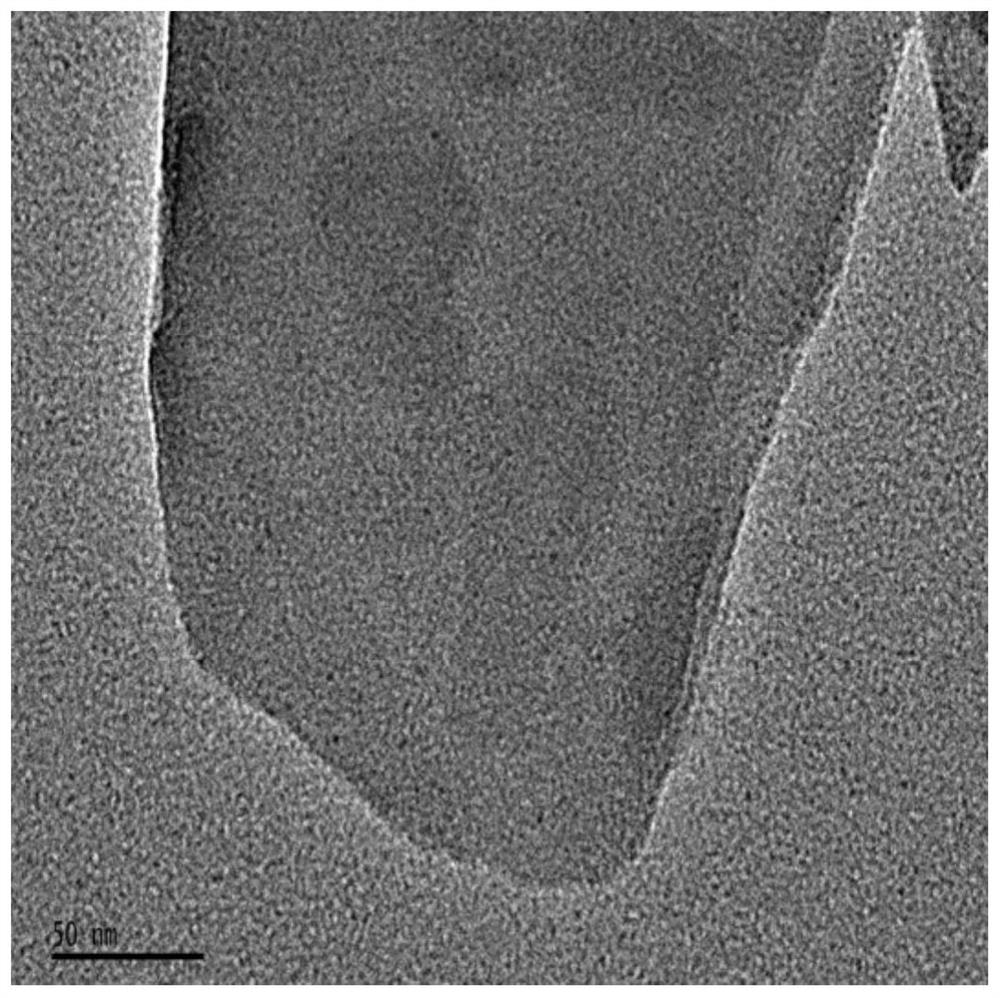
A kind of vanadyl phosphate catalyst, its preparation method and application
A technology of vanadyl phosphate catalyst and vanadium source, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc. It can solve problems such as low conversion rate, loss cost of additive elements, and complicated preparation process. achieve good crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The embodiment of the present invention provides a kind of preparation method of vanadyl phosphate catalyst, it comprises the following steps:
[0067] S01: Put the vanadium source in a container, add a mixture of deep eutectic solvent: isobutanol: benzyl alcohol (volume ratio) = (0.15~0.25): (3~5): 1, and heat up to 100 ° C ~ 180 ℃ for 2 hours to 8 hours, then cooled to 30 ℃ to 80 ℃, then added phosphorus source, heated to 100 ℃ to 200 ℃ to continue the reaction for 10h to 24h, and the product was filtered, washed and dried to obtain the vanadyl phosphate precursor. The molar ratio of the phosphorus in the phosphorus source and the vanadium in the vanadium source is (0.8~1.5): 1, and the concentration of the described vanadium pentoxide in isobutanol and benzyl alcohol mixed solution is 0.02g / mL~0.12g / mL mL, the mass ratio of the vanadium source to the deep eutectic solvent is (50~10):1
[0068] S02: Calcining the vanadyl phosphate precursor at a temperature of 350° C...
Embodiment 1
[0077] (1) Weigh 15g V 2 o 5 Place in a 250mL three-necked flask, add a mixture of 1.5g polyethylene glycol-thiourea deep eutectic solvent, 80mL isobutanol and 20mL benzyl alcohol, stir and mix evenly, reflux at 135°C for 3 hours and then cool to 60°C ℃.
[0078] (2) Slowly add 11.29mL of 85% H 3 PO 4 , and continued to reflux for 16 hours at a temperature of 135° C. Filter and wash with absolute ethanol to obtain a blue precipitate, and dry it in air at 120° C. for 24 hours to obtain a vanadyl phosphate catalyst precursor powder.
[0079] (3) The obtained vanadyl phosphate catalyst precursor powder was pressed into tablets under a pressure of 15 MPa, crushed, and 20-40 mesh catalyst particles were sieved.
[0080] (4) Afterwards, the catalyst particles were activated in situ from room temperature to 430°C at a rate of 2°C / min for 12 hours in an atmosphere of n-butane / oxygen / nitrogen reaction gas with a volume ratio of 1.5:17:81.5 to obtain phosphoric acid vanadyl cataly...
Embodiment 2
[0084] (1) Weigh 15g V 2 o 5 Place in a 250mL three-neck flask, add a mixture of 1.5g polyethylene glycol-urea deep eutectic solvent, 80mL isobutanol and 20mL benzyl alcohol, stir and mix evenly, reflux at 135°C for 3 hours and then cool down to 60°C .
[0085] (2) Slowly add 11.29mL of 85% H 3 PO 4 , and continued to reflux for 16 hours at a temperature of 135° C. Filter and wash with absolute ethanol to obtain a blue precipitate, and dry it in air at 120° C. for 24 hours to obtain a vanadyl phosphate catalyst precursor powder.
[0086] (3) The obtained vanadyl phosphate catalyst precursor powder was pressed into tablets under a pressure of 15 MPa, crushed, and 20-40 mesh catalyst particles were sieved.
[0087] (4) Afterwards, the catalyst particles were activated in situ from room temperature to 430° C. at a rate of 2° C. / min under a reaction gas atmosphere for 12 hours to obtain a vanadyl phosphate catalyst.
[0088] Detection:
[0089] Take by weighing vanadyl phos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com