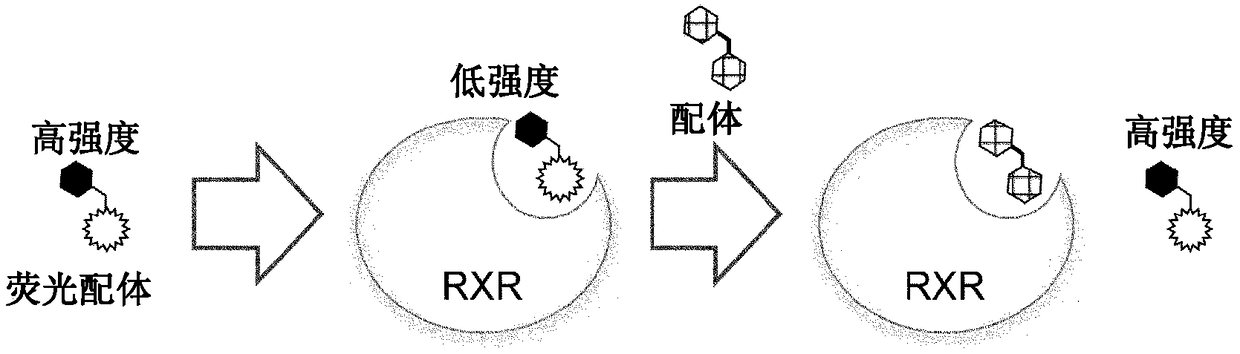
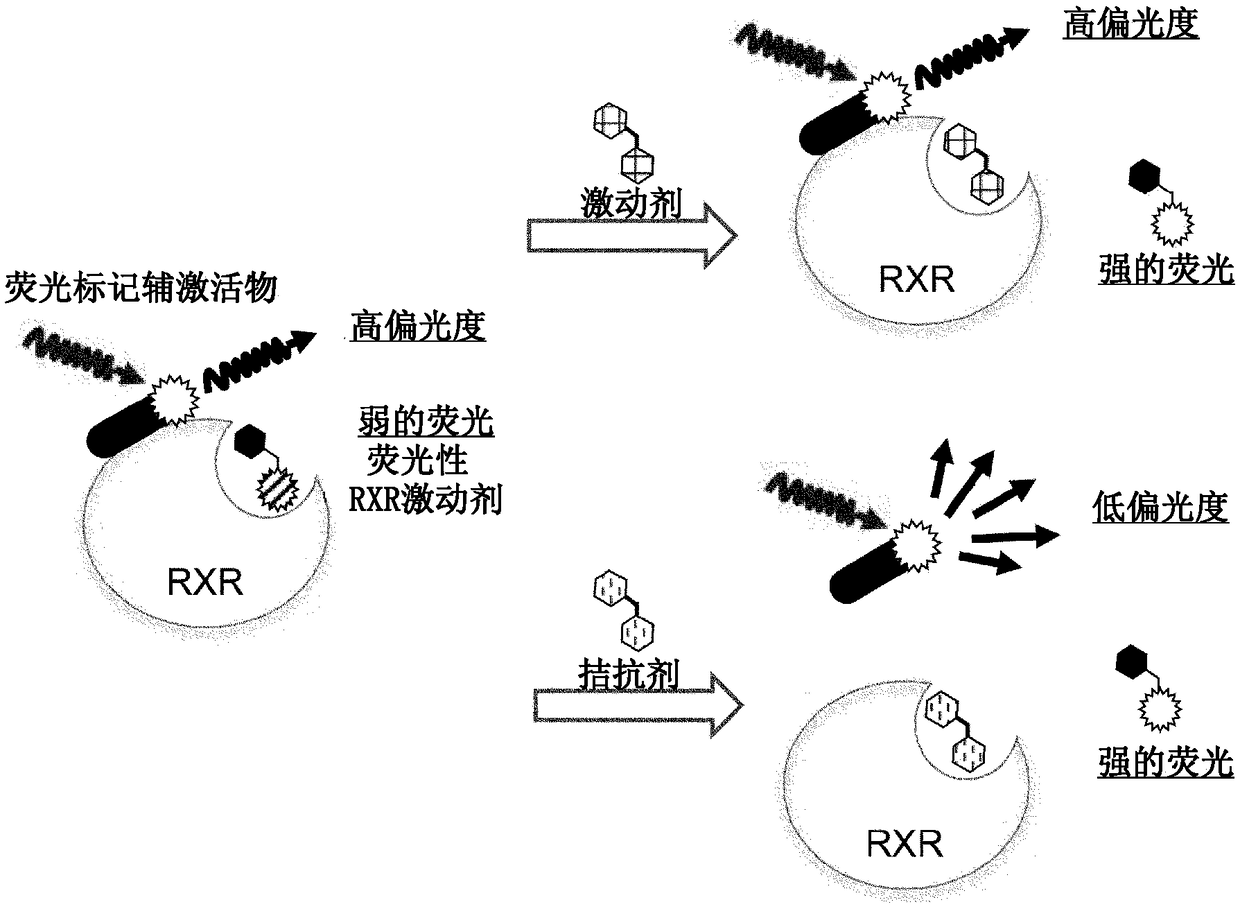
Fluorescent ligand for retinoid x receptor, and application thereof
A receptor binding and binding technology, which is applied in the direction of organic active ingredients, medical preparations containing active ingredients, active ingredients of heterocyclic compounds, etc., can solve the problem of increased RXR amount, inability to study excitatory or antagonistic properties, and shortened working time. long wait question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
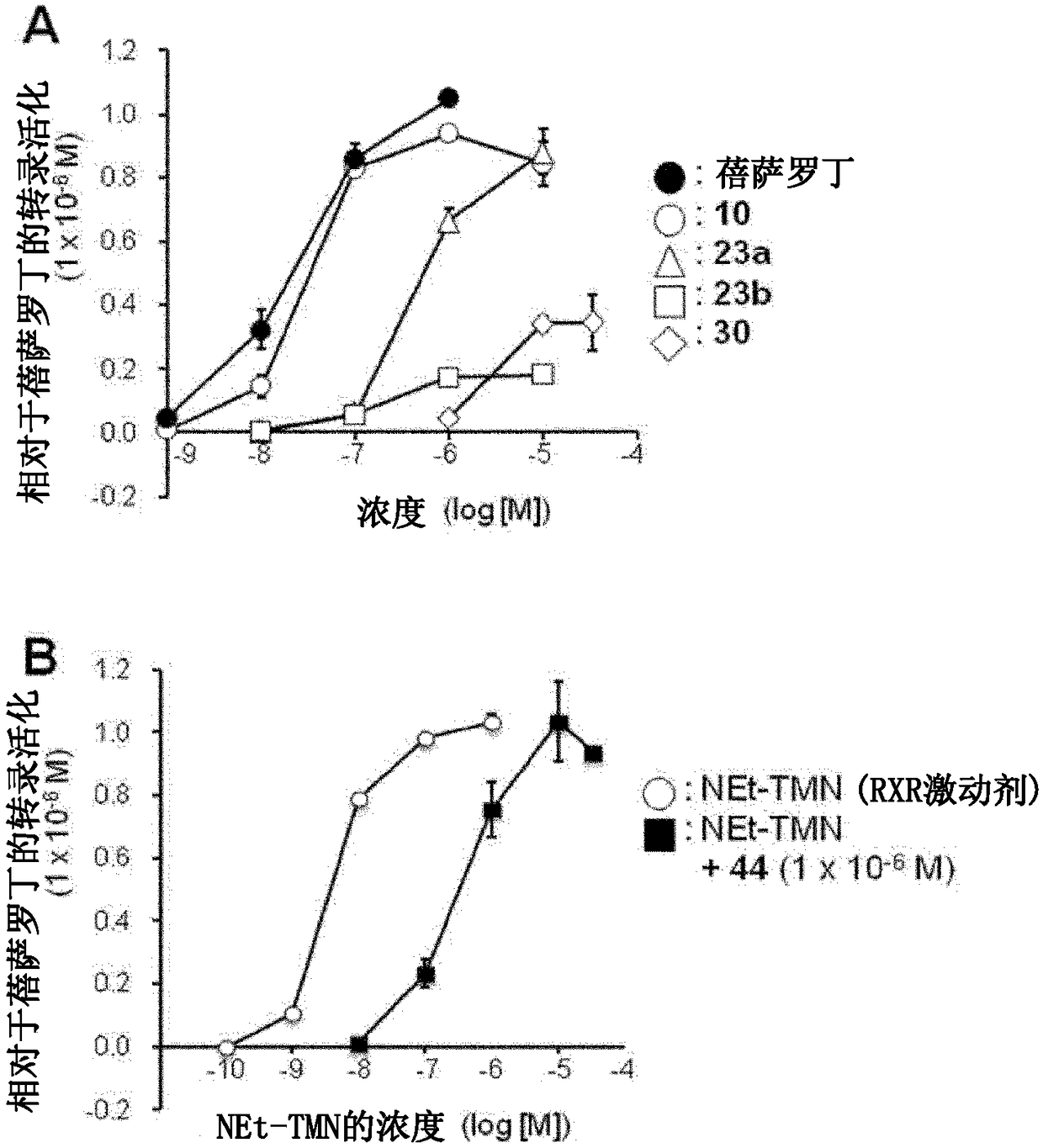
[0104] [Example 1] Synthesis of target compound 10
[0105] The synthesis scheme of the target compound 10 in this example is shown in the following formula.
[0106]
[0107] 1) Synthesis of compound 2
[0108] 2,4-Dihydroxybenzaldehyde (3.3 g, 24 mmol) was dissolved in acetic acid (24 mL), and bromine (1.2 g, 24 mmol) was added dropwise over 10 minutes, followed by stirring at room temperature for 20 hours. Thereafter, it was confirmed that the reaction was complete using a TLC (Thin Layer Chromatography) plate (ethyl acetate:n-hexane=1:3, developed twice). Water (35 mL) was added, and the precipitated solid was collected by filtration and washed with water to obtain crude crystals (5.3 g). Flash column chromatography (ethyl acetate:n-hexane=1:4→1:3), followed by recrystallization (ethyl acetate:n-hexane), gave light brown needle-like crystals of 2 (1.5g, 28%) .
[0109] 1 H NMR (300MHz, CDCl 3 )δ11.25(s,1H),9.70(d,1H,J=0.5Hz),7.66(s,1H),6.63(s,1H),6.11(s,1H)
[01...
Embodiment 2
[0133] [Example 2] Synthesis of target compound 23a-c
[0134] The synthetic schemes of the target compounds 23a-c in this example are shown below.
[0135]
[0136] 1) Synthesis of compound 18
[0137]2,5-Dimethyl-2,5-hexanediol (2.0 g, 14 mmol) was dissolved in concentrated hydrochloric acid (20 mL), and stirred at room temperature for 14 hours. The solid was collected by filtration, washed with water, dissolved in dichloromethane (150 mL), and washed with water (50 mL). After drying the organic layer with magnesium sulfate, the solvent was distilled off under reduced pressure to obtain a crude product (2.4 g) of a white solid. The obtained crude product and 2-bromophenol (2.1 mL, 20 mmol) were dissolved in anhydrous dichloromethane (30 mL), aluminum trichloride (170 mg) was added, and the mixture was stirred at room temperature under an argon atmosphere for 3 hours. The completion of the reaction was confirmed by a TLC plate (ethyl acetate:n-hexane=1:10), the reaction...
Embodiment 3
[0179] [Example 3] Synthesis of target compound 30
[0180] The synthesis scheme of the target compound 30 in this example is shown in the following formula.
[0181]
[0182] 1) Synthesis of Compound 25
[0183] Thymol (150 mg, 1.0 mmol) was dissolved in methanol (4 mL), silver sulfate (370 mg, 1.2 mmol) and iodine (300 mg, 1.2 mmol) were added, and the mixture was stirred at room temperature for 6 hours. Use (ethyl acetate:n-hexane=1:10) to confirm the completion of the reaction, pour the reaction solution into saturated sodium thiosulfate water (30 mL), filter with celite, and extract with ethyl acetate (30 mL×3). The organic layer was washed with water (40 mL×2) and saturated brine (40 mL). The obtained organic layer was dried over magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain a crude product (270 mg) of a yellow solid. Flash column chromatography (ethyl acetate:n-hexane=1:50→1:30) gave 25 (230 mg, 85%) as a yellow solid.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com