Crystal form B of apatinib, and preparation method and application thereof
A technology of apatinib and crystal form, which is applied in the field of apatinib crystal form B and its preparation, can solve problems such as affecting drug efficacy, and achieve the effects of excellent high-humidity stability and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 5
[0028] The preparation of embodiment 1 to 5 Apatinib B crystal form
[0029] Weigh 1g of apatinib raw material (purchased from Shanghai Hanxiang Biotechnology Co., Ltd., purity>98%) and place it in a container, add the solvents (analytical grade) in Table 1 respectively, and stir or shake at room temperature for 24h , and then centrifuged at 12,000 rpm for 5 minutes. The solid in the lower layer was removed and dried in vacuo to obtain a white solid, which is the Apatinib B crystal form. Weigh and calculate its yield, and the results are shown in Table 1.
[0030] Table 1 Preparation of Apatinib Form B
[0031]
Embodiment 6
[0032] Example 6 Characterization of Apatinib Form B by XRPD
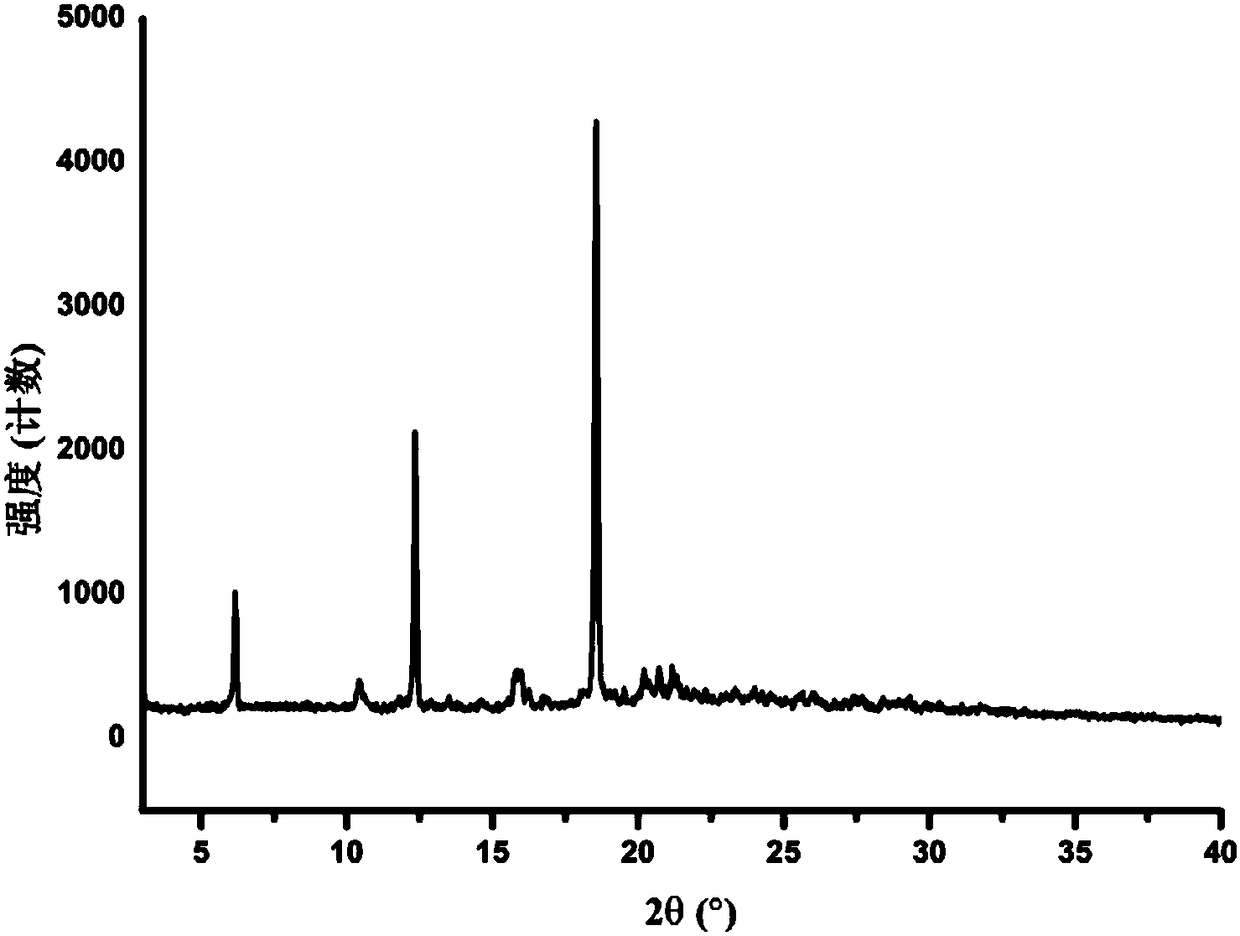
[0033] The measurement of the X-ray powder diffraction (XRPD) pattern is carried out at room temperature using a D8Advance X-ray diffractometer from Bruker, Germany. The specific collection information is as follows: The radiation source is Cu-Kα 1 Ray, scan range (2θ range) from 3° to 40°, scan speed 12° / min, scan step size 0.02, slit width 0.01. Samples were processed using glass slides pressed directly onto the test plate. Subsequent XRPD patterns all adopt similar measurement methods.
[0034] Determination of the XRPD spectrum of the Apatinib B crystal form prepared according to the method described in Example 1 has characteristic Cu-Kα at 2θ=6.17, 10.66, 12.35, 14.62, 18.56, 20.21, 20.84, 21.21° 1 Diffraction peaks, such as figure 1 shown. The error range of 2θ value is ±0.2°. After testing, the error range of the 2θ value can also be ±0.15°.
[0035] A specific list of characteristic X-ray diffraction ...
Embodiment 7
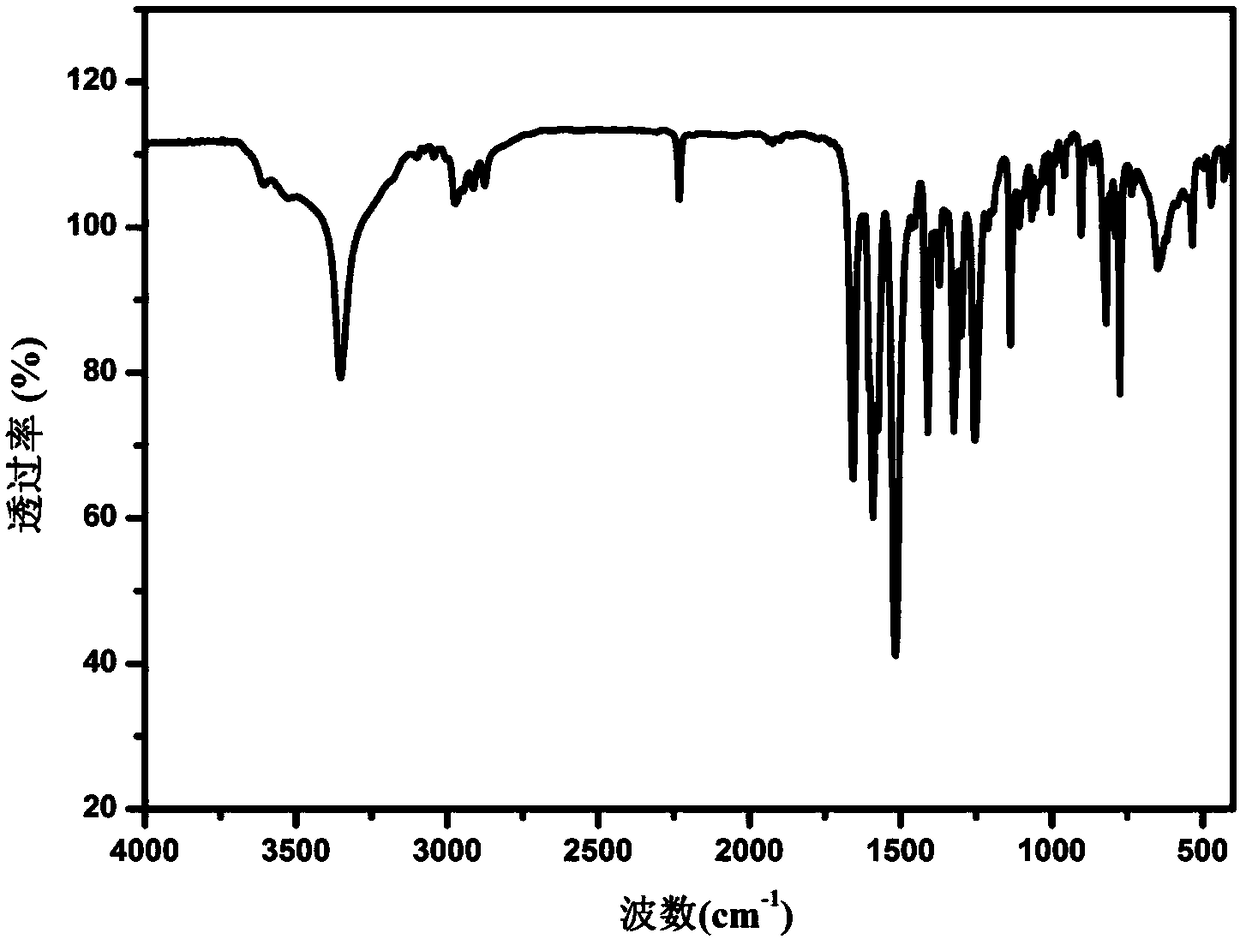
[0040] Example 7 Characterization of Apatinib B Crystal Form by Infrared Spectroscopy
[0041] Use the Nicolet-Magna FT-IR 750 infrared spectrometer of Nicolet Corporation of the United States to detect the infrared spectrum of the Apatinib B crystal form prepared according to the method described in Example 1 at room temperature, and the detection range is: 4000~350cm -1 . The obtained infrared spectrum is attached as figure 2 As shown, at 3352, 2232, 1656, 1590, 1515, 1253, 1136cm -1 There is a strong absorption peak. Detect the Apatinib B crystal form prepared according to the method described in Example 2-5, the obtained infrared spectrum and the attached figure 2 The spectra shown are essentially the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com