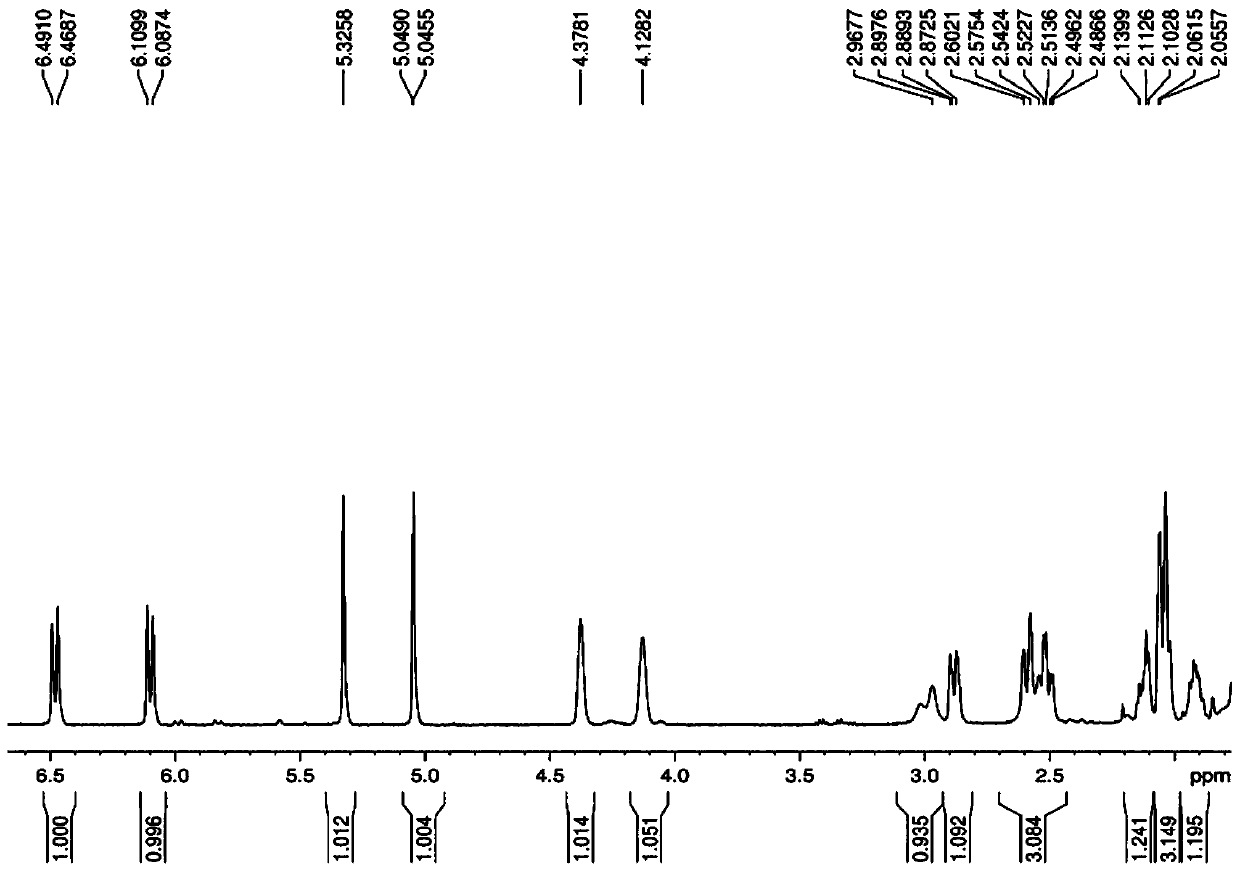
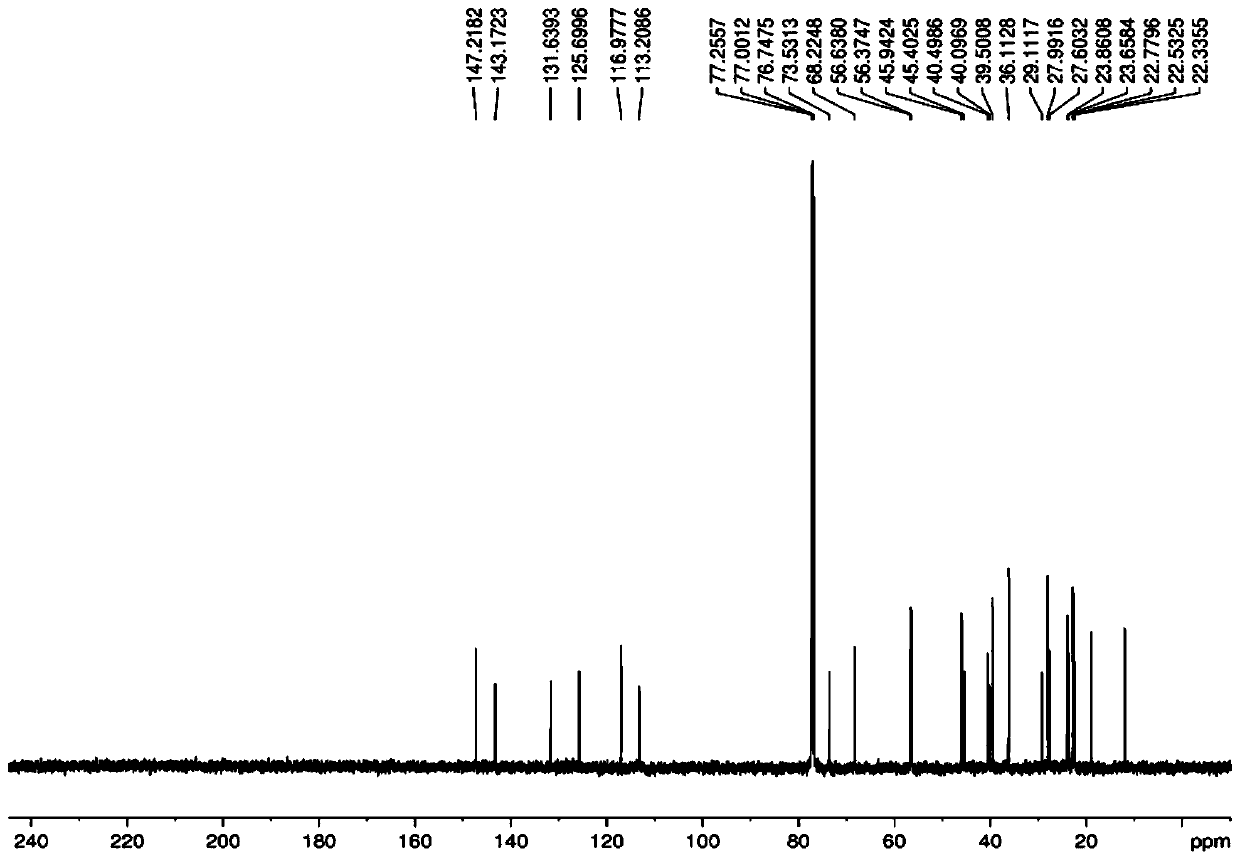
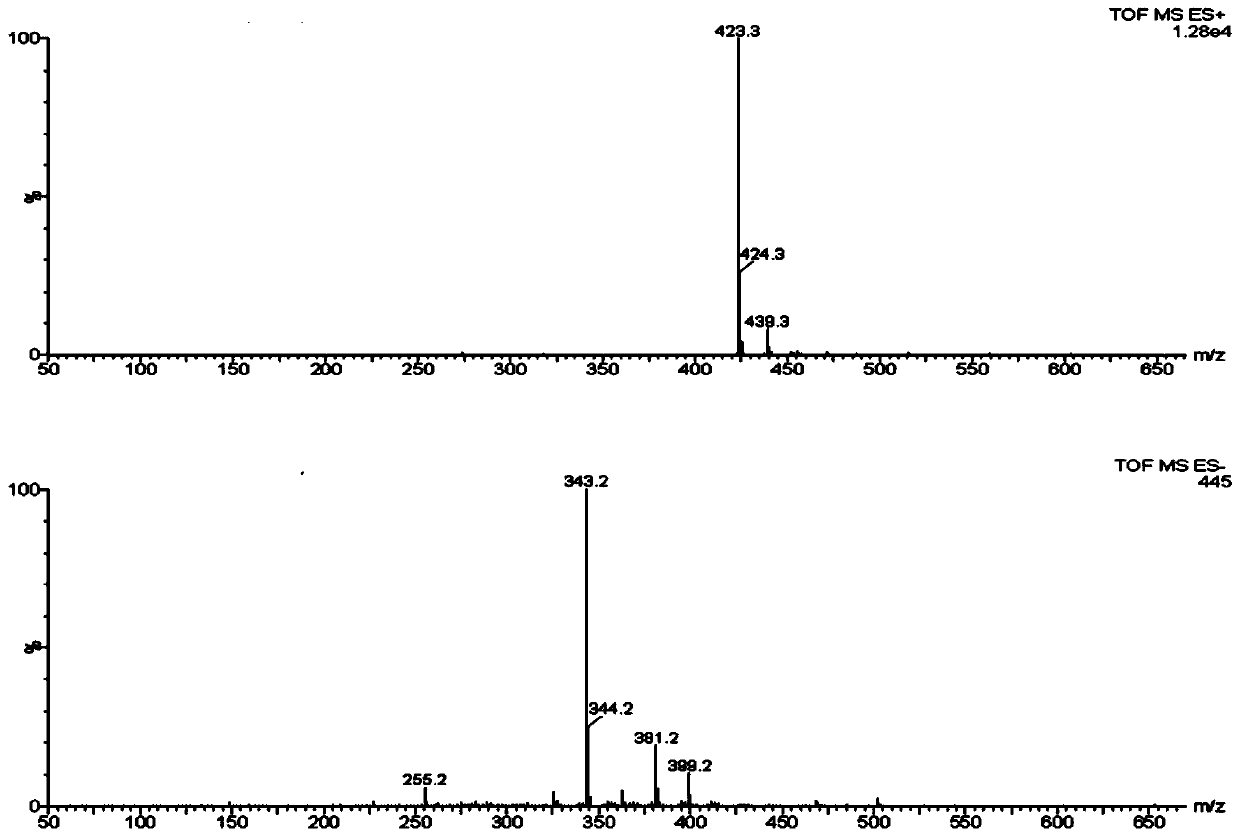
A kind of directional synthesis method and application of isomer impurity of alfacalcidol
A technology of alfacalcidol and isomers, applied in the field of chemical pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 formula 4 compound
[0068] Add the compound of formula 2, 80g of vitamin D3, 5L of dichloromethane, and 35g of imidazole into the reaction flask, keep away from light, and add 47g of tert-butyldimethylsilyl chloride dropwise at 0-5°C; React at 30°C, monitor by TLC until the reaction of the raw materials is complete, wash with water, stir and dry with anhydrous sodium sulfate, and obtain a solution of the compound of formula 3; cool the solution of the compound of formula 3 in methylene chloride to below -20°C, and stir in the dark, Add 300 g of sulfur dioxide gas, monitor by TLC until the reaction of the raw materials is complete, concentrate under reduced pressure, and concentrate the mother liquor in vacuo to obtain a brown oily substance, namely 115 g of the compound of formula 4.
Embodiment 2
[0069] The preparation of the compound of embodiment 2 formula 5
[0070] Add 115g of the compound of formula 4, 140g of sodium bicarbonate, and 1.5L of 95% ethanol into the reaction flask, keep away from light, protect with argon, heat to reflux for about 2-3h, monitor by TLC until the reaction of the raw materials is complete, concentrate, and add acetic acid to the residue 1.5 L of ethyl ester and 1.5 L of water were stirred until the solids were completely dissolved, left to stand, separated, the organic layer was washed twice with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain a light yellow transparent oil, namely 100 g of the compound of formula 5.
Embodiment 3
[0071] The preparation of embodiment 3 formula 6 compound
[0072] Add 2L of dichloromethane, 1.5L of acetonitrile, 100g of the compound of formula 5, and 90g of N-methylmorpholine oxide into the reaction bottle, heat to reflux, add 20g of selenium dioxide, reflux for 2-3h, monitor by TLC, and cool to room temperature , adding 1.5 L of water, stirring and standing for liquid separation, the organic phase was washed twice with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain a dark brown oily substance, namely 101 g of the compound of formula 6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com