Preparation method of 3,3',4,4'-tetraaminobibenzene
A technology of tetraaminobiphenyl and nitrobenzene, which is applied in the field of preparation of chemical intermediates, can solve problems such as difficult handling, serious pollution, and difficult industrial production, and achieve low environmental pollution, high quality, and simple post-processing. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Take 106g of o-chloronitrobenzene and 32g of 43% liquid caustic soda, add 0.4g of 1,4-naphthoquinone and 20g of water, raise the temperature to 56°C, add 314g of 43% liquid caustic soda and 224g of formaldehyde in double drops, react for 7 hours, then add 250g water, cooled to room temperature, and filtered. Add 0.94g sodium dodecylbenzenesulfonate, 0.56g 1,4-naphthoquinone, 0.94g aluminum-nickel alloy, 226g water, 55.2g 43% liquid caustic soda to the filter cake, stir and heat up to 56°C, then add hydrazine hydrate dropwise 50g, the dropwise addition time is 7h, after the addition, continue to keep warm for 0.5h. After the reaction, 100ml of cold water was added, cooled to room temperature, dried by suction filtration, and recrystallized with absolute ethanol to obtain a reduced product (reduced material). Stir 124g of concentrated hydrochloric acid and 34.3g of water to cool down to 5°C, slowly add a batch of reducing materials, keep at 5°C for 2h, then raise the tem...
Embodiment 2~8
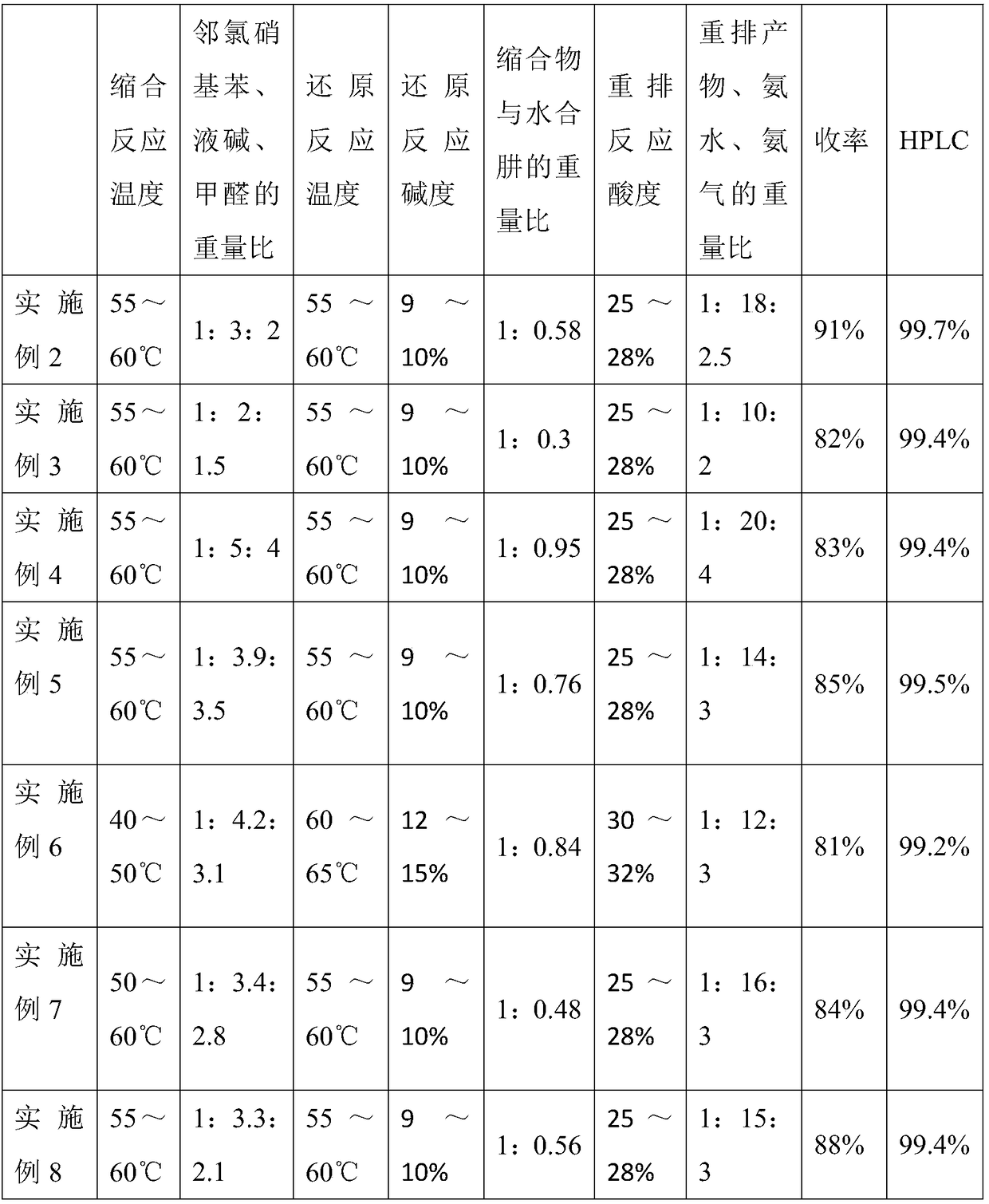
[0030] The reaction process is as in Example 1, changing process parameters, such as condensation reaction temperature, o-chloronitrobenzene, liquid caustic soda, weight ratio of formaldehyde, reduction reaction temperature, weight ratio of condensate to hydrazine hydrate, rearrangement product, ammonia water, ammonia 3,3',4,4'-tetraaminobiphenyl was prepared according to the weight ratio of gas, and the IR of the product was consistent with the standard spectrum of 3,3',4,4'-tetraaminobiphenyl. The result of embodiment 2~8 is as shown in table 1:
[0031] Table 1
[0032]
[0033] The invention reduces environmental pollution while improving product quality, makes product production clean and environmentally friendly, and makes the product more suitable for mass production.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com