Drug release system, azithromycin eye preparation containing drug release system, and preparation method of drug release system
A technology for azithromycin and ophthalmic preparations, which is applied in the field of drug release system and azithromycin ophthalmic preparations including its components, and can solve the problems of inability to stay active ingredients, lack of sustained-release effect, and increase the pain of patients, and achieve good intraocular Penetration, easy availability of raw materials, and the effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A preparation method of a release system of azithromycin ophthalmic preparation, the steps are: take water for injection, add polycarbophil solution under stirring, after swelling, take water for injection, add citric acid to dissolve citrate Sodium citrate, poloxamer 407, stir to dissolve, mix with the above polycarbophil solution, adjust the pH value to 6.0-6.5, stir well, steam sterilize at 121°C, and cool to room temperature.
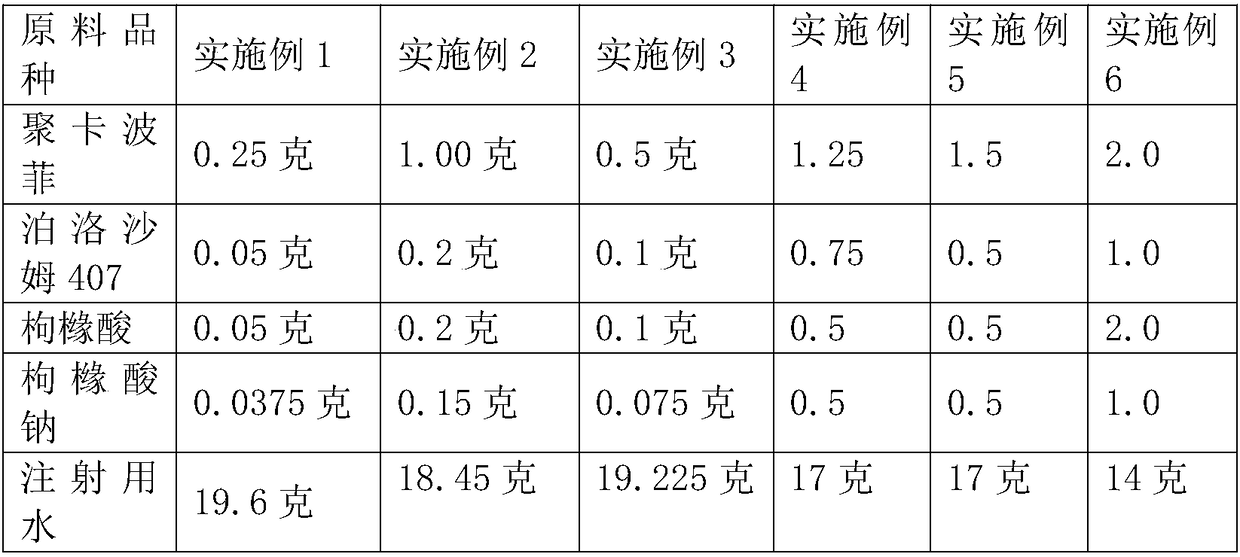
[0029] Table 1 Preparation of Azithromycin Ophthalmic Preparations Drug Release System Raw Material Components and Consumption
[0030]
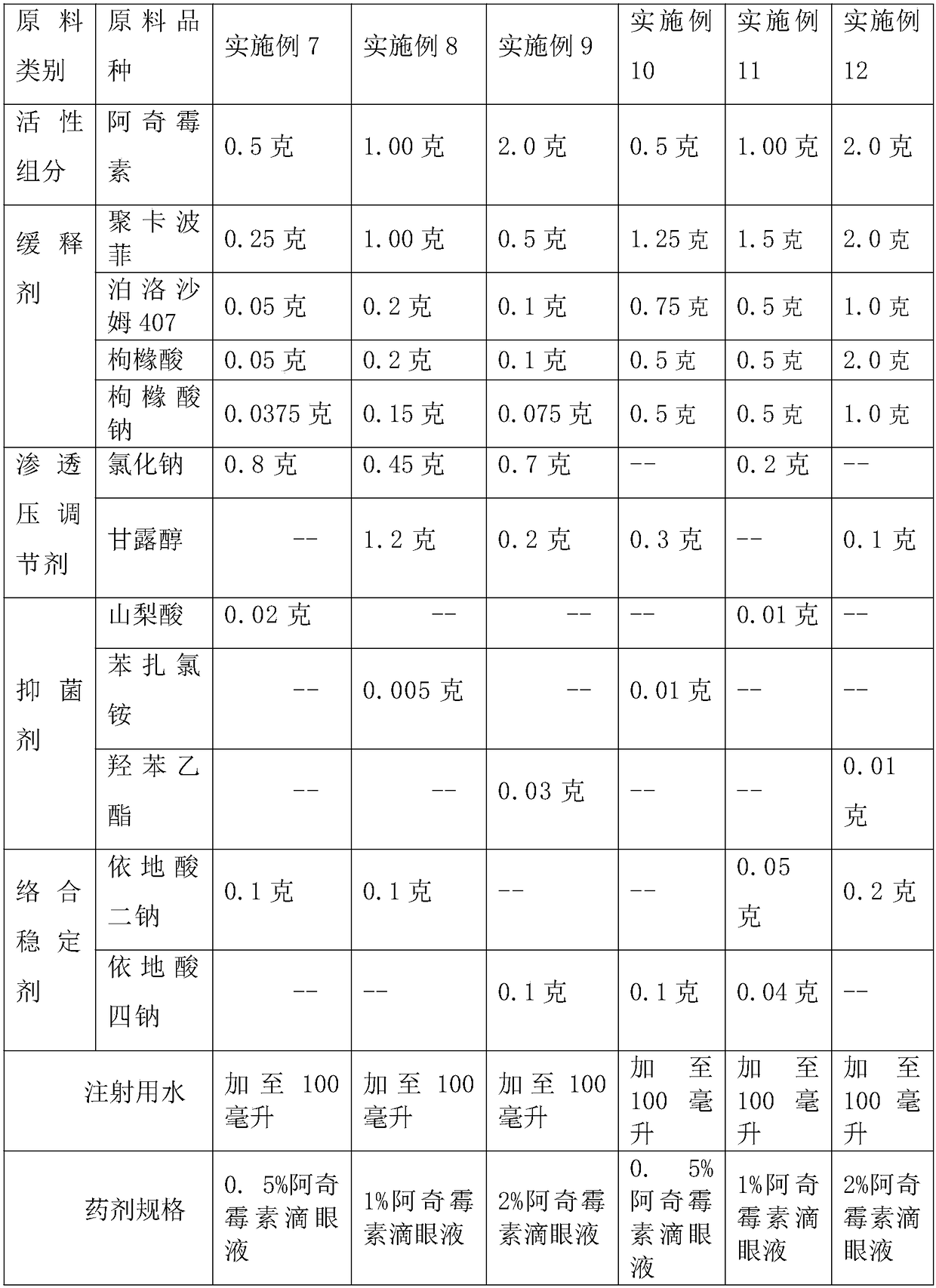
[0031] A slow-release azithromycin ophthalmic preparation comprising a drug delivery system for azithromycin ophthalmic preparations, which uses azithromycin as a medicinal raw material, and uses the drug delivery system polymer of the azithromycin ophthalmic preparation as a mucoadhesive polymer drug delivery system, Every 100 parts by weight of the preparation contains 0.5-2 parts by weight of azithrom...
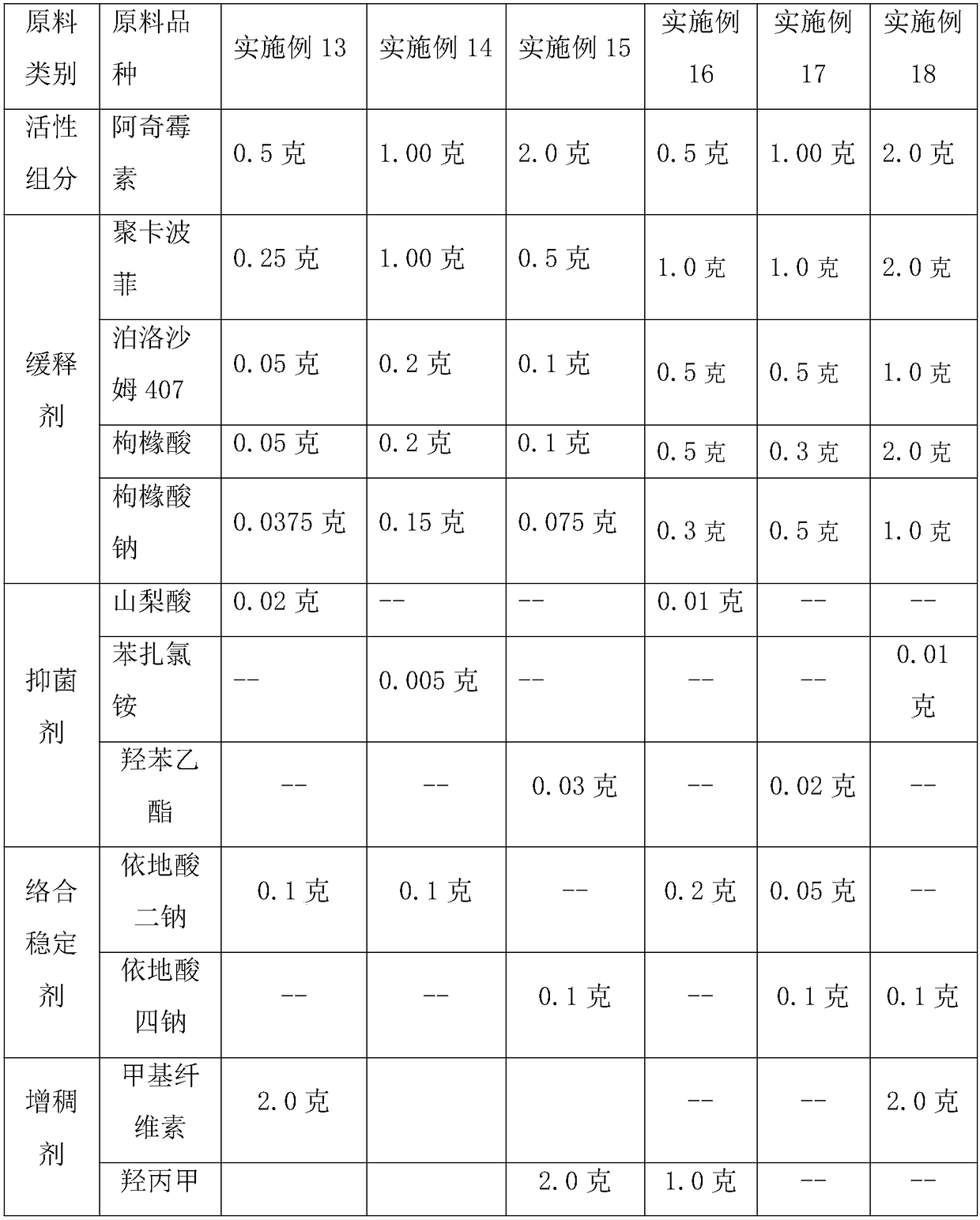
Embodiment 13-18
[0050] Table 3 Example 13-18 Preparation of Sustained-release Azithromycin Ophthalmic Gel Raw Material Components and Consumption
[0051]
[0052]
[0053] The preparation method is as follows: take 2 / 5 of the prescribed amount of water for injection, add the prescribed amount of polycarbophil under stirring, let it swell for more than 2 hours, then take a small amount of water for injection, add the prescribed amount of citric acid to dissolve sodium citrate, poirot Sham 407, stir to dissolve, mix with the above polycarbophil solution, add 2mol / L sodium hydroxide solution, adjust the pH value to 6.0-6.5, stir well, steam sterilize at 121°C for 20 minutes, and cool to room temperature Add the prescribed amount of azithromycin; take a small amount of water for injection to dissolve the thickener and let it disperse and let it cool; and dissolve the prescribed amount of complexation stabilizer and bacteriostat with water for injection, stir to dissolve and then add the dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com