Markable dark red fluorescent active ester
A technology of active ester and deep red, which is applied in the direction of fluorescence/phosphorescence, luminescent materials, and material analysis through optical means, can solve the problems that have not been reported on deep red fluorescent probes, and achieve excellent photostability, stable photochemical properties, The effect of high absorption coefficient and quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
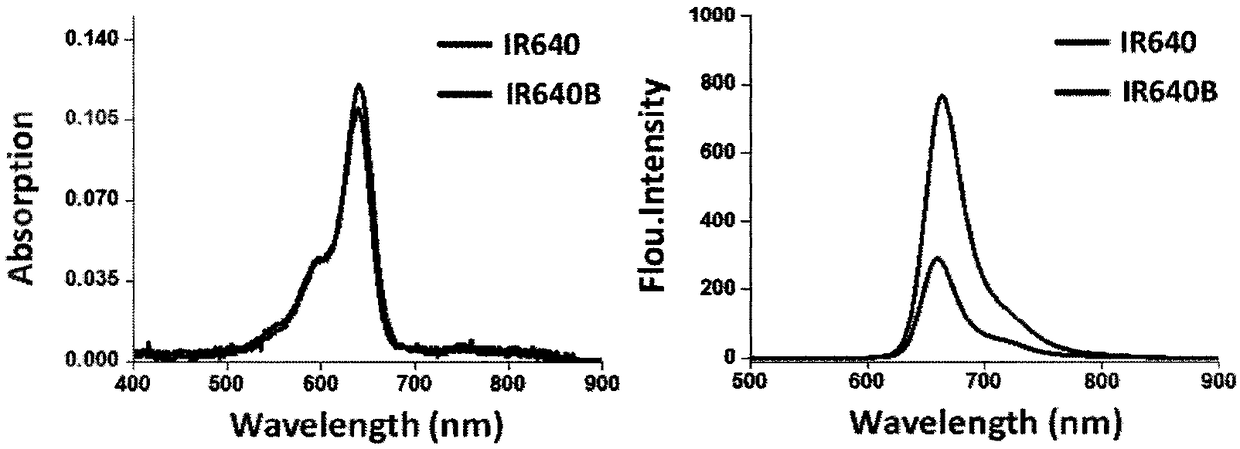
Image
Examples
Embodiment 1
[0058] Synthesis of compound 1:
[0059]
[0060] Mucbromic acid (5.94g, 23.8mM) was dissolved in 40mL ethanol, aniline (4.29g, 4.2mL, 46.1mM) was diluted with 20mL ethanol, and then the diluted aniline solution was added dropwise to the ethanol solution of mucbromic acid , about 10 minutes. The reaction vessel needs to be reacted under the conditions of vigorous stirring and 40°C. After the aniline is added, carbon dioxide will be generated, and the reaction is not indicated until the generation is complete. Then 50 mL of glacial ether was added, and a bright yellow solid precipitated. Filter, wash with glacial ether, and dry to obtain the product.
Embodiment 2
[0062] Synthesis of compound 2:
[0063]
[0064] Dissolve 2.3.3-trimethylindole (2.00g, 12.6mM) and butysulphonolactone (5.60g, 41.2mM) in 5.0mL o-dichlorobenzene, stir in an oil bath at 120°C, and reflux for 12h After cooling to room temperature, it was dropped into 450 mL of diethyl ether to precipitate, and the crude product was obtained by filtration. After being dissolved in water, it was extracted three times with chloroform to obtain an aqueous layer solution, which was freeze-dried to obtain pure sulfoindole.
Embodiment 3
[0066] Synthesis of compound 3:
[0067]
[0068] Compound 2 (0.413g, 1.40mM), compound 1 (0.267g, 0.700mM) and anhydrous sodium acetate (0.116g, 1.41mM) were dissolved in 13mL of acetic anhydride, and heated in an oil bath at 70°C for 3h. After the reaction was completed and cooled, diethyl ether precipitated to obtain a solid product. The product was purified by column chromatography on cassia gum (100-120 mesh), and the product was collected by gradient elution of methanol: dichloromethane (0-20%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com